- Visibility 76 Views
- Downloads 7 Downloads
- DOI 10.18231/j.ijmr.2019.054
-
CrossMark
- Citation
Study to assess quality of antimicrobial use by point prevalence survey at a tertiary care centre
- Author Details:
-
B Usha
-
K Sudha
-
N Shanmuga Vadivoo *
Introduction
Antimicrobial agents are among the most frequently prescribed drugs and Antimicrobial resistance is more prevalent in hospital settings due to unrestricted and improper use of antimicrobials[1],[2] Approximately one third of patients admitted to hospital receive antibiotics during their hospital stay[3],[4] and reports say that up to 50% of all courses of antimicrobial therapy are deemed unnecessary.[3],[4],[5] Antimicrobial resistance is of great concern also because it is associated with increased patient ’s duration of hospitalisation, expenditure, and fatality rate.[6]
Since both frequent and prolonged use of antimicrobial agents may promote the emergence of resistance,[7],[8] antimicrobial stewardship is recommended as a means of reducing antimicrobial resistance, along with lowering the risk of adverse drug events, treatment complicati ons, and institutional costs. [9],[10] It is important for institutions to understand their patterns of antimicrobial use to identify appropriate stewardship interventions that have the greatest likelihood of impacting institutional antimicrobial utilization and therefore, aforementioned consequences of antibiotics use. Analysing the antimicrobial use pattern can provide statistics from which explicit antimicrobial stewardship interventions can be targeted.
Several studies have assessed the relationship between differences in prescribing patterns and the occurrence of resistance in bacterial isolates. These data have provided background for the introduction of antibiotics restriction and cycling of antibiotics to fight for the emergence of antibiotic resistance[8],[10],[11]. Based on an ecological study in Europe, countries with higher antibiotic consumption had correspondingly higher rates of anti biotics resistance, highlighting the fact that uncontrolled prescription of antibiotics is a key risk factor for resistance.[8] Further support for this link was evident by the decrease of resistance with reduced prescription of broad-spectrum antibiotics, which was also associat ed with a cost-saving outcome.[10]
Many factors can lead to fluctuations and misuse in antibiotic use, such as over-prescription of broad-spectrum antibiotics and inappropriate treatment of likely viral respiratory tract infections. [12] Indeed, the lack of prescriber’s perception of the effect of antibiotics on the emergence of resistance remains a key driver of inappropriate antimicrobial use and rates of resistance.[10]
The world’s largest consumer of antibiotics for human health was India, as reported in 2010 and which happens to be 12.9 x 109 units (10.7 units per person).[13] The Government of India has issued a National Policy for Containment of Antimicrobial Resistance which promotes antimicrobial use surveillance in the community and hospitals. To begin with, the Government proposed the drug utilization studies of antimicrobials in hospitals. Also, it suggests that the data on consumption trends can be used for intervention studies to promote the rational use of these medicines. Hence fundamental components of the stewardship program are to measure the quantity & Quality of antibiotic use.
Defined daily doses (DDD) of prescribed antimicrobials per 100 bed days are a good quantitative measure of antimicrobial consumption. And point prevalence survey in a structured way helps in the qualitative assessment of Antimicrobial use at a given point of time. The main disadvantage of the quantitative approach is if it can actually measure the quality of antibiotic prescribing. Hence PPS can help to target the areas for quality improvement in antibiotic prescribing, establish interventions to promote better stewardship of antibiotics to assist in fight against antimicrobial resistance and a praise the validity of interventions through periodic surveys. A PPS serves as a convenient, inexpensive surveillance system of antimicrobial consumption, as opposed to continuous surveillance.
PPS is an established stewardship tool in few countries, but in some parts of the world, clinicians have just begun to understand and explore how to use it. There are very few Indian studies on Point prevalent survey of antibiotic use, and to the best of our knowledge, our study is probably the first from South India.
In this study, a point prevalence survey was conducted to measure antimicrobial utilization and patterns of use at a tertiary care teaching hospital for inpatients. For future point-prevalence surveys we hope to use this information as baseline data. Also, the effect of antimicrobial stewardship interventions will be determined, and it will bring out awareness among prescribers regarding appropriate AU.
Aims and Objectives
To determine the prevalence of patients receiving at least one antimicrobial agent and the prevalence of individual antibiotics prescription from specific Wards & ICU on the survey date.
To outline the antimicrobial prescribing pattern (choice of antimicrobial agent, indication, route and, duration of therapy).
To study relationships existing between Empiric/Definitive AU for CAI/ HAI and Surgical antibiotics prophylaxis with relevance to the anatomic site.
Materials and Methods
The point prevalence survey was conducted at 670 bedded teaching hospitals in South India. This hospital provides tertiary medical care in twenty-eight wards, including three ICUs spanning paediatric, medical and surgical units.
Study design
A Point prevalence study
Study period
One week period to assess rational antibiotic s prescriptions by Point prevalence study during the last week of January 2019
Study tool
In the house, simple PPS tool was prepared based on point prevalence survey methodology on antibiotic use in hospitals from WHO- version 1.1 as a reference tool.[14],[15]
Data collection
A multidisciplinary survey team conducted the PPS and collected the relevant data at selected units. The survey team was trained on how to aggregate the information before the start of the survey. Briefly, the training session lead by the principal investiga tor who introduced survey personnel to the aims & objectives of the study; the purpose for collection of each item on the data collection tool including terms and indicator codes definitions; methodology for evaluating individual patient data; and each survey personnel roles and responsibilities. For a period of one week, the point prevalence survey was conducted by the survey team within 8 hours from 8 am to 4 pm daily. The survey team performed data collection using a PPS tool (based on WHO methodology) which comprised of a patient-level structured template to document antibiotics use on the survey date. The team also reviewed admitted patients’ treatment charts and recorded antibiotics used on the survey date. Patients’ demographics like age, sex, admitted ward, and total numbers of patients on admission on survey day were noted. Other appropriate information such as administered antibiotics, its route of administration, dosages, dosing intervals were also included. Addition al information recorded w as information on patients’ clinical diagnosis and antibiotic use indications.
Indication for AU
As defined by WHO[14],[15] Indication for AU was noted for hospital-or community-acquired infections (HAI, CAI) or for Surgical/Medical antibiotic prophylaxis (SAP /MAP) or Unknown. The team re viewed signs & Symptoms from medical and nursing case records and other relevant charts to identify if the indication is a HAI/CAI/SAP/MAP.
The survey team’s decision if a patient was infected (HAI/CAI) was based on clinical grounds and WHO PPS guideline definitions. Actively infected patients were diagnosed by the presence of signs and symptoms on the survey date. Also, they were considered infected when the patient was still receiving treatment for that infection on the date of the survey even when signs and symptoms were no longer present. Clinical diagnosis of hospital-acquired infections describes infections occurring 48h after admission and categorized as community-acquired if occurring within 48h of admission.
Antimicrobial use for CAI/HAI was identified as being definitive or empiric. Definitive treatment was defined “when the patient underwent antibiotic treatment following the identification of either a site of infection or isolation and reporting of infecting pathogenic microorganism”. Empiric treatment was defined as “one that was started for a presumed or possible infection without a site or infecting organism being identified”.
Prophylaxis was defined as “the use of an antimicrobial agent to prevent infection when an infection was not already present”. Treatment indication was categorized as unknown “if there was no identifiable reason for antimicrobial use after review of the medical records”. The classification of drugs was based on WHO anatomical therapeutic classification (ATC) of medicines
Targeted antimicrobials
Includes Beta lactams, fluoroquinolones, third -generation cephalosporins (3GC), carbapenems, aminoglycosides, vancomycin, and piperacillin-tazobactam.
Inclusion criteria
Patients who were receiving at least one systemic antibacterial, on the day of the survey.
Exclusion criteria
Patients undergoing same-day treatment or surgery
Emergency room patients who were not yet admitted
Palliative care patients
Ophthalmology ward s and dermatology ward patients were also excluded from the survey because mostly these patients will be treated with Topical antibiotics.
Orders for anti-retroviral, anti-tuberculosis, antifungal and anti-parasitic medications were also excluded from the survey.
Results
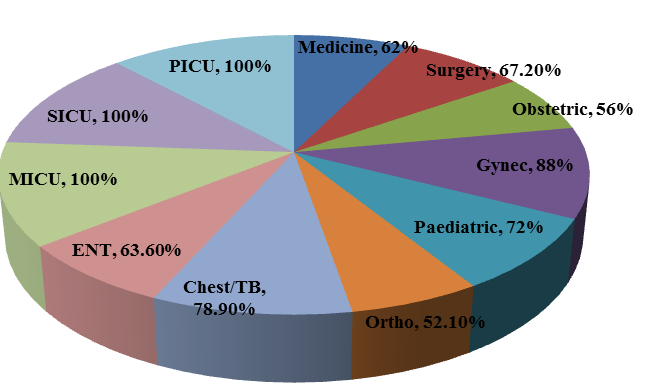
Total of 502 admitted patients w ere included in the study. Among the 502 patients, prevalence rate of antibiotic use in different wards & ICU ’s was 64.7 %(325 patients). All the ICU patients (100%) were on at least one antibiotic and the highest rate of antibiotic use was in Gynaecology ward (88.0 %) when compared to other wards [Figure 1]. More than one antibiotic was administered for 45% of patients and switch of IV to Oral antibiotics was reported in 27.69% of patients. The mean age of the patients on antibiotic orders (SD) was 39.
Indications for Antibiotic use in the surveyed locations were shown in [Figure 2] and 51.4 % indication was for CAI followed by surgical prophylaxis (30.2%). This also compares the empiric and definitive indication for CAI and HAI in different wards &ICU’s. Both CAI and HAI were mostly treated empirically rather than by targeted therapy. The overall major class of antibiotics used were third generation Cephalosporin’s ( 3GC)-44%, foll owed by Penicillins (14.4%) and Metronidazole (12%) as shown in [Table 1]. and the most common antibiotic prescribed was Cefotaxim (36.9%). Across all specialties, the most common preferred choice of antibiotic used was 3GC with nearly half of the admitted patients receiving 3-GC. A significant proportion of prescribed antibiotics (69%) were administered via the parenteral route. The highest rate of parenteral antibiotics was used in Paediatrics’ and ICU’s. Distribution of surgical antibiotic prophylaxis by anatomic site infections is shown in [Table 2] and 3GC was the prevalent antibiotics used as surgical prophylaxis for all anatomic sites

| S. No | Ward | Medicine | Surgery | Obg | Gynec | Paed | Ortho | Chest/tb | Ent | Micu | Sicu | Picu | Overall | % |
| 1 | Amino Glycosides | 6 | 22 | 0 | 0 | 4 | 12 | 1 | 0 | 0 | 3 | 1 | 49 | 9.04 |
| 2 | Penicillin | 23 | 0 | 4 | 0 | 28 | 4 | 12 | 6 | 0 | 1 | 0 | 78 | 14.4 |
| 3 | 1st and 2nd generation Cephalosporins | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1.85 |
| 4 | 3rd Generation Cephalosporins | 43 | 81 | 22 | 16 | 36 | 24 | 6 | 3 | 4 | 2 | 2 | 239 | 44.1 |
| 5 | Beta lactam+ Beta lactamase inhibitors | 0 | 8 | 0 | 0 | 0 | 8 | 0 | 0 | 1 | 1 | 0 | 18 | 3.32 |
| 6 | Metronidazole | 6 | 10 | 22 | 18 | 0 | 5 | 0 | 0 | 1 | 2 | 1 | 65 | 12 |
| 7 | Fluroquinlones | 8 | 14 | 1 | 2 | 2 | 9 | 1 | 0 | 0 | 0 | 2 | 39 | 7.2 |
| 8 | Carbapenems | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 5 | 0.92 |
| 9 | Tetracyclines | 8 | 0 | 0 | 7 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 18 | 3.32 |
| 10 | Linezolids | 0 | 2 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 10 | 1.85 |
| 11 | Macrolides | 4 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 1.11 |
| 12 | Nitrofurantoin | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 0.92 |
| total | 104 | 148 | 51 | 43 | 70 | 74 | 21 | 9 | 6 | 10 | 6 | 542 | 100 |
| S.No | ANTIBIOTICS | GI* | UTI* | SSTBJI* | GO* | ENT* | Total | % |
| 1 | Amikacin | 4 | 6 | 11 | 0 | 0 | 21 | 12.9% |
| 3 | Gentamicin | 4 | 0 | 1 | 0 | 0 | 5 | |
| 4 | Amoxicillin | 0 | 0 | 0 | 0 | 2 | 2 | 5.4% |
| 5 | Ampicillin | 0 | 0 | 4 | 0 | 0 | 4 | |
| 6 | Augmentin | 0 | 0 | 1 | 4 | 0 | 5 | |
| 7 | Cephalexin | 2 | 0 | 0 | 0 | 0 | 2 | 1.97% |
| 8 | Cefixime | 2 | 0 | 0 | 0 | 0 | 2 | |
| 9 | Cefotaxime | 25 | 7 | 27 | 30 | 2 | 91 | 52.2% |
| 10 | Ceftriaxone | 2 | 0 | 4 | 0 | 0 | 6 | |
| 11 | Cefaperazone +Sulbactam | 1 | 1 | 3 | 0 | 0 | 5 | |
| 12 | Ciprofloxacin | 0 | 0 | 4 | 0 | 0 | 4 | 3.4% |
| 13 | Levofloxacin | 0 | 0 | 0 | 3 | 0 | 3 | |
| 14 | Imipenem | 0 | 2 | 1 | 0 | 0 | 3 | 1.5% |
| 15 | Doxycycline | 1 | 2 | 0 | 0 | 0 | 3 | 1.5% |
| 16 | Linezolid | 0 | 0 | 1 | 0 | 0 | 1 | 0.5% |
| 17 | Metrogyl | 8 | 0 | 6 | 28 | 0 | 42 | 20.8% |
| 18 | Piperacillin -Tazobactam | 0 | 2 | 0 | 0 | 0 | 2 | 0.9% |
| Total | 49 | 20 | 63 | 65 | 4 | 201 |
Discussion
Point prevalence surveys on AU may be considered a simple method of monitoring the effectiveness of antibiotic policies and of providing useful data on patterns of antibiotic use, thus informing and guiding local and national antibiotic stewardship.
The aim of the present study was to evaluate Antibiotics prescribing patterns and to identify areas for further work where improved use of antibiotics needs to be addressed. Good outcomes with antimicrobials (i.e. appropriate antimicrobial prescribing and reduction of resistance to antimicrobials) require the use of antimicrobial stewardship approaches and completion of PPS at regular intervals[16].
PPS survey in this study revealed that the prevalence of patients who received at least one antimicrobial agent was 64.74%. Community-acquired infection was the most common indication for antibiotic use followed by surgical prophylaxis [Figure 2] . A significant percentage of antibiotics (69 %) were administered via Intra venous route. The highest use of parenteral antibiotics was found in ICU’s and Paediatrics wards.
We report a higher rate of AU when compared with two national studies - 51.6% & 61.5%,[17],[18] Ghana (51%)[19] Canada (29%) [20], Egypt ( 59%)[21] , Turkey (54.6%) [22], Italy (47%) [23], Australia (46%) [24], the United Kingdom (40.9%) [25], Latvia (39%) [26] & United States (33% ) .[27] However, it was lower than in Kenya (67.7%),[28] Iran (66.6%)[29] and China (78.2%).[30] In Consistent with other studies, we also observed that the majority of the antimicrobial usage was for therapeutic purposes rather than prophylaxis .[26],[27],[29],[31]
Major proportions of antibiotics were mainly used for the treatment of community-acquired infections (51.4%) and surgical prophylaxis (30.2%) as shown in [Figure 2] . Prevalence rate reported in this study is comparable to an Indian study [32] which reports 55.6% of antibiotics used for CAI & 18.9% for Prophylaxis. Our data is high when compared to another Indian study where is 36.8% for CAI and 35% for prophylaxis and 40% CAI and 33% SAP as reported in Ghana [19] . In contrast, reports from Egypt [21] and Kenya[30] show that most common indication was for SAP ( 38.4%) and MAP (29%) respectively.
On reviewing empirical antimicrobial therapies with CAI, the two most common antimicrobials were 3GC(38%) followed by Ampicillin (15%). The antimicrobial regimens prescribed for surgical prophylaxis include 3GC (48.2%), Metronidazole (42%) and Aminoglycosides (13%). This is probably the first study describing antimicrobial use in South India using point prevalence survey.
The most commonly prescribed antibiotics in this study were 3GCs. Two other Indian studies that reviewed antibiotic prescription practices among hospitalized children in two private hospitals in central India reported similar findings.[33],[18]
In Concordance with our findings, 3GCs were the most commonly prescribed antimicrobials in many studies from different geographical locations like Eastern Europe (35.7%) and Asia (28.6%) in the global ARPEC (Antibiotic resistance & prescribing in European children) study [31], Turkey (18.4%) [22], Italy (20%)[23], Latvia (28%),[26] & Iran (43.5%).[30] However, studies from The United Kingdom & Australia report penicillin plus enzyme inhibitor combinations as the most commonly prescribed antimicrobials.[24],[25] Carbapenem prescription in this study was lower than in Turkey (12.7%) [22], Italy (6%)[23] Iran (5.2%)[29], Australia (3.2%) [24], but higher than in Latvia (0.5%)[26].
The current guidelines recommend the use of third-generation Cephalosporins only when first-line agents are ineffective [34]. Hence this study identified an opportunity to improve antimicrobial use by prescribing first line drugs for hospitalized CAI patients.
A recent study analysing the antibiotic sensitivity of the major bacterial pathogens isolated from community-acquired pneumonia in India indicated high susceptibility to first-line agents (Ampicillin and amoxicillin-clavulanic acid) [35]. This study reinforces that third-generation cephalosporins can be avoided as first-line therapy for respiratory CAIs.
According to CDDEP(Center for disease dynamics, economics & Policy) antimicrobial resistance surveillance data [36] obtained both from all age groups, in 2017, 77% of the Eshericia coli isolates were resistant to 3GC in India, which was much higher than in Australia (11%), the United Kingdom (11%), Argentina (17%), the United States (13%), South Africa (19%), and China (63%) [36]. Exposure of 3GC in children could lead to earlier colonization, which facilitates the spread of Extended Spectrum Beta Lactamase producing bacteria among family members. This leads to a further increase in prevalence of ESBL Enterobacteriaceae infections in India and subsequently the consumption of Carbapenems. Our findings in a study published in 2018 indicate the need for increased compliance with national guidelines [37].
Therefore, antimicrobial stewardship initiatives targeting these indications may have the greatest potential to impact on patient care, antimicrobial use, the incidence of nosocomial infections, and resistance. Another area for antimicrobial stewardship intervention, as suggested in previous surveys is with antimicrobial surgical prophylaxis.
The second most common indication for antibiotic administration reported was surgical prophylaxis accounting for 31.4% of all antibiotic prescriptions. Also, our observation that 3GCs were the most commonly used antibiotics for surgical prophylaxis is not in par with the published international guidelines which recommends the use of first-and second-generation cephalosporins instead of third-generation cephalosporins [38]. A detailed review of surgical prophylaxis prescriptions revealed that the selection, timing, and duration of administration were frequently inconsistent with the evidence-based practices. Approximately three fourth of patients receiving surgical prophylaxis, were treated with SAP for greater than 24 h at the time of this survey. First-generation Cephalosporins and Penicillins were used for SAP which accounted for 7.4 % of all antibiotic agents even though these are the recommended agents for a large proportion of surgical procedures [38]. And 52.2% of surgical prophylaxis prescriptions were from Broad-spectrum agents such as 3GC and beta-lactam plus beta-lactamase inhibitor combinations, which might contribute to increase in multidrug-resistant organisms’ emergence. In response to these findings, our hospital has proposed a quality improvement project focused on improving surgical site infection prevention practices, including surgical antibiotic prophylaxis.
This study has important limitations inherent to the use of point prevalence surveys like a single point in time and so the results can be affected by normal day-to-day variation, existing trends, and seasonality of antimicrobial use. Since they are more likely to overlap the study date, differences in patient population and prescribing practices should be considered when comparing these results to other institutional point prevalence surveys. But given the availability of national treatment guidelines and similarities in prescribing practices among academic teaching hospitals, the results of this survey provides a good initial estimation of institutional antimicrobial use in South India.
Few Clinicians are aware of National antibiotic use guidelines and even if they are aware, adherence to antibiotic guidelines (compliance rate) is low. To improve rational antibiotics use, such guidelines need to be incorporated in Hospital antibiotic policies and translated in to practice. This may result in a reduction in hospital antibiotic consumption & its associated complications.
Conclusion
This prospective point prevalence survey provided important baseline information on antimicrobial use and identified potential targets for future antimicrobial stewardship initiatives at our tertiary care teaching hospital. This study reports a high prevalence of antibiotic use among inpatients with a relatively high prevalence among General medicine & paediatrics patients. There is a high use of 3GC&Metronidazole and low percentage use of higher antibiotics like Meropenem. Majority of the antibiotics use indications were community -acquired infections and surgical prophylaxis.
Source of Funding
None.
Conflict of Interest
None.
References
- . . . [Google Scholar]
- S K Fridkin, C D Steward, J R Edwards. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis: Official Publ Infect Dis Soc Am 1999. [Google Scholar]
- C M Kunin, T Tupasi, W A Craig. Use of antibiotics. A brief exposition of the problem and some tentative solutions. Ann Int Med 1973. [Google Scholar]
- F Ansari, M Erntell, H Goossens, P Davey. The European surveillance of antimicrobial consumption (ESAC) pointprevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis: Official Publ Infect Dis Soc Am 2009. [Google Scholar]
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Int Med 2003. [Google Scholar]
- Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis: Official Publ Infect Dis Soc Am 2006. [Google Scholar]
- S L Bronzwaer, O Cars, U Buchholz. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis 2002. [Google Scholar]
- H Goossens, M Ferech, R Vander Stichele, M Elseviers, E P Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005. [Google Scholar]
- Dellit TH, Owens RC, McGowan Jr JE. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis: Official Publ Infect Dis Soc Am 2007. [Google Scholar]
- C Bantar, B Sartori, E Vesco. A hospital wide intervention program to optimize the quality of antibiotic use: impact on prescribing practice, antibiotic consumption, cost savings, and bacterial resistance. Clin Infect Dis: Official Publ Infect Dis Soc Am 2003. [Google Scholar]
- Costelloec. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. Br Med J 2010. [Google Scholar]
- Wise. Antimicrobial resistance: is a major threat to public health. Br Med 1998. [Google Scholar]
- T P Van Boeckel, S Gandra, A Ashok, Q Caudron, B T Grenfell, S A Levin. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014. [Google Scholar] [Crossref]
- . . . [Google Scholar]
- . World Health Organization. WHO methodology for point prevalence survey on antibiotic use in hospitals, version 1.1. World Health Organization . [Google Scholar]
- G M Taani. Longitudinal point prevalence survey of antibacterial use in Northern Ireland using the European Surveillance of Antimicrobial Consumption (ESAC) PPS and Global PPS tool. Epidemiology Infection 2018. [Google Scholar]
- Gandra. Point prevalence surveys of antimicrobial use among eight neonatal intensive care units in India. Int J Infect Dis 2016. [Google Scholar]
- M Sharma, A Damlin, A Pathak, C Stalsby Lundborg. Antibiotic prescribing among pediatric inpatients with potential infections in two private sector hospitals in Central India. PLoS ONE 2015. [Google Scholar]
- Appiah- Labi. Antimicrobial resistance and Infection control. 2018. [Google Scholar]
- C Lee. Point prevalence survey of antimicrobial utilization in a Canadian tertiary-care teaching hospital. J Epidemiol global health 2015. [Google Scholar]
- MahaTalaat. A Point Prevalence Survey of Antibiotic Use in 18 Hospitals in Egypt . Antibiotics 2014. [Google Scholar]
- M Ceyhan, I Yildirim, C Ecevit, A Aydogan, A Ornek, N Salman, A Somer, N Hatipoglu, Y Camcioglu, E Alhan. Inappropriate antimicrobial use in Turkish pediatric hospitals: A multicenter point prevalence survey. Int. J. Infect. Dis 2010. [Google Scholar]
- De Luca, M, D Dona, C Montagnani, A Lo Vecchio, M Romanengo, C Tagliabue. Antibiotic prescriptions and prophylaxis in Italian children: Is it time to change? Data from the ARPEC project. PLoS ONE 2016. [Google Scholar]
- Joshua Osowicki. Australia-wide point prevalence survey of the use and appropriateness of antimicrobial prescribing for children in hospital. MJA 2014. [Google Scholar]
- Gharbim. Using a simple point prevalence survey to define appropriate antibiotic prescribing in hospitalized children across the UK BMJOpen. 2016. [Google Scholar]
- I Sviestina, D Mozgis. Antimicrobial usage among hospitalized children in Latvia: A neonatal and pediatric antimicrobial point prevalence survey. Med 2014. [Google Scholar]
- A L Pakyz, H E Gurgle, O M Ibrahim, M J Oinonen, R E Polk. Trends in antibacterial use in hospitalized pediatric patients in United States academic health centers. Infect Control Hosp Epidemiol 2009. [Google Scholar]
- C Okoth. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya findings and implications. Findings Implic 2018. [Google Scholar]
- A Fahimzad, Z Eydian, A Karimi, F Shiva, S Sayyahfar, M Kahbazi, G Soleimani. Surveillance of antibiotic consumption point prevalence survey 2014: Antimicrobial prescribing in pediatrics wards of 16 Iranian hospitals. Arch Iran Med 2016. [Google Scholar]
- . D Xie. A multicenter point-prevalence survey of antibiotic use in 13 Chinese hospitals. J Infect Public health 2015. [Google Scholar]
- A Versporten, J Bielicki, N Drapier, M Sharland, H Goossens. The worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: Developing hospital-quality indicators of antibiotic prescribing for children. J. Antimicrob. Chemother 2016. [Google Scholar]
- Gandra. Antibiotics. 2017. [Google Scholar]
- Sanjeev Singh T.J, Versporten, A, Sengupta, S, Fini, P, Sharland, M, K R Kumar. A point prevalence surveillance study from pediatric and neonatal specialty hospitals in India. J Pediatr Infect Dis 2014. [Google Scholar]
- . . National Center for Disease Control. National Treatment Guidelines for Antimicrobial Use in InfectiousDiseases 2016. [Google Scholar]
- D Torumkuney, R Chaiwarith, W Reechaipichitkul, K Malatham, V Chareonphaibul, C Rodrigues. Results from the survey of antibiotic resistance (SOAR) 2012-14 in Thailand, India, South Korea and Singapore. J Antimicrob Chemother 2016. [Google Scholar]
- . Center for Disease Dynamics. Economics & Policy. Available online . [Google Scholar]
- N.Shanmuga Vadivoo, B Usha. ESKAPE pathogens: Trends in antibiotic resistance pattern. Med Pulse Int J Microbiol 2018. [Google Scholar]
- D W Bratzler, E P Dellinger, K M Olsen, T M Perl, P G Auwaerter, M K Bolon. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect 2013. [Google Scholar]
How to Cite This Article
Vancouver
Usha B, Sudha K, Vadivoo NS. Study to assess quality of antimicrobial use by point prevalence survey at a tertiary care centre [Internet]. Indian J Microbiol Res. 2025 [cited 2025 Sep 03];6(3):245-252. Available from: https://doi.org/10.18231/j.ijmr.2019.054
APA
Usha, B., Sudha, K., Vadivoo, N. S. (2025). Study to assess quality of antimicrobial use by point prevalence survey at a tertiary care centre. Indian J Microbiol Res, 6(3), 245-252. https://doi.org/10.18231/j.ijmr.2019.054
MLA
Usha, B, Sudha, K, Vadivoo, N Shanmuga. "Study to assess quality of antimicrobial use by point prevalence survey at a tertiary care centre." Indian J Microbiol Res, vol. 6, no. 3, 2025, pp. 245-252. https://doi.org/10.18231/j.ijmr.2019.054
Chicago
Usha, B., Sudha, K., Vadivoo, N. S.. "Study to assess quality of antimicrobial use by point prevalence survey at a tertiary care centre." Indian J Microbiol Res 6, no. 3 (2025): 245-252. https://doi.org/10.18231/j.ijmr.2019.054
