Introduction
Biological preparations are thermolabile products and unstable during storage. This fluctuation may lead to a decrease in safety and efficacy of these products. During the preparation of a vaccine, there may be some proteins and other macromolecules that can be sensitive to heat, humidity, light and other environmental conditions or it may interact with packing material or other products used in vaccine. The chemical reactions like solvolysis, oxidati on, reduction, racemization etc. that occur in pharmaceutical products may lead to formation of degradation of products, loss of potency of active pharmaceuticals ingredients (API), loss of excipient activity like antimicrobial preservative action and antioxidants etc.1 After determining these relationships, enhancing the stability from production to administration to patient is an important part of vaccine preparation. As time passes since production, the reduction in potency may occur gradually. The stability of a pharmaceutical product can also be affected because of microbial changes like growth of microorganisms in non-sterile products and changes in preservative efficacy.2
Stability may be defined as the capability of a formulation in a specific container/closure to remain within its physical, chemical, microbiological, toxicological, protective and informational specifications.3 Fluctuations in handling and storage conditions may exert temperature stresses that leads to significant changes in stability profile. The stability standards of a vaccine must be calculated analytically through testing. The handling and storage conditions be illustrated to ensure the minimum levels of potency, identity and purity continue to be met over the stated shelf life of a vaccine. The development of cold chain requirements has become a new approach to deal with temperature sensitivity of vaccines. Thus, stability testing evaluates the effect of environmental factors on the quality of a drug substance or a formulated product which is utilized for prediction of its shelf life, determine proper storage conditions and suggest labelling instructions. Moreover, the data generated during stability testing is an important requirement for regulatory approval of a drug or formulation.4
The modern vaccine formulation development path from the discovery of an immunogen to a usable vaccine includes: (1) physical and chemical characterization of the antigenic component, (2) development of stability-indicating assays including potency, (3) evaluation and optimization of the route of administration and adjuvants (in both animal models and in clinical trials), and (4) formulation design to maximize the candidate vaccine's (antigen and adjuvant) stability, shelf life and immunogenic potential. A major focus of vaccine formulation development, in many cases, is the enhancement of potency through the use of vaccine adjuvants, since many candidate immunogens fail to transfer from the laboratory to the patient due to suboptimal efficacy in humans. One key approach to increase the success rate for new vaccine candidates is thus to ensure the appropriate formulation in the presence of conventional and/or novel adjuvants. The purpose of this study is to hike perception about the scientific and technical challenges confronting to successfully formulate and stabilize different types of vaccines, both in terms of stability of antigens, adjuvants and their complexes.
Materials and Methods
The current research has been conducted in mutual consent of the university of Lahore and Ottoman Pharma, Lahore. The whole research was executed in the research and development (R&D) department of Ottoman Pharma, Lahore. In pharmaceutical industry samples of each batch of every product are retained at specified temperature up to one year of its date of expiry and humidity for future reference. The focus of this study is to utilize these retained expired samples for the long lasting stability of Otto Flu Plus Vaccine.
Source of vaccine
Total number of five retained samples was collected from the retained samples in refrigerator placed in QC department of Ottoman Pharma. Each vial was properly labeled and stored at 4°C. The details of each sample are given below:
Table 1
Detail of Otto Flu Plus Vac retained samples used in current study
Source of broilers
Total of 25- day old broilers were purchased from hatchery of Big Bird poultry breeders and shifted to the clean and fumigated experimental animal house of Ottoman Pharma. All the birds were offered with feed and water ad libitum under same environmental conditions.
Experimental design
Real time stability testing
Total number of five retained samples was collected from the retained samples refrigerator placed in QC department. Each vial was analyzed at different time period of 3 months, 6 months, 9 months, 12 months and 15 months. Each vial was analyzed for its physicochemical properties (Table 1).
Accelerated stability testing
Five vials of 300 ml of Otto Flu Plus oil based vaccine were further transferred into sterilize vials in such a way that every vial has 100 ml of vaccine. Each of the vials was properly labeled according to master sheet (Table 2). Master Vial of 300 ml was de-sealed in sterile bio safety cabinet and transferred to vials containing 100 ml each. Each vial was transferred to incubator pre-set at different temperature as 4℃, 8℃, 12℃, 16℃ and 20 ℃. Vaccine was evaluated for its physiochemical stability such as density, viscosity, pH, particle distribution, stability and in vivo determination of anti-Influenza HI antibody titer after storage for 2 months, 4 months and 6 months. The details of each vaccine are given in (Table 2).
Efficacy testing
Total of 30 birds were divided into four groups each containing 4 birds. The birds were marked with specific color and immunized with respective vaccine at different time interval. The blood of each bird of every group was collected from wing vein at 28-day post vaccination. The serum was extracted and subjected for anti-Influenza HI antibody titer.
Haemagglutination inhibition titer
U-shaped bottom microtitre plates of 96 well were labelled appropriately. 50 µl of normal saline was dispensed in 1st row of 96 well plate upto 12th well with the help of microtitre pippete. 50 µl of antigen was added to the first well of appropriately numbered column. 2-fold serial dilution were made by transferring 50ss µl serum from first well of numbered columns to successive wells. Added 50 µl of 4HA virus antigen in each of the well upto 11th well and incubated for 30 minutes at 37℃. 50 µl of 1% washed RBC were added to all wells. Plat es were gently tapped and kept at 37 ℃ for 30 minutes.
Statistical analysis
The data obtained in the study was analyzed by mean standard deviation and subsequently through repeated measure analysis of variables (ANOVA) using SPSS version 21.
Table 2
Master data sheet of labels
Results
Effect of accelerated temperature on viscosity at different time interval
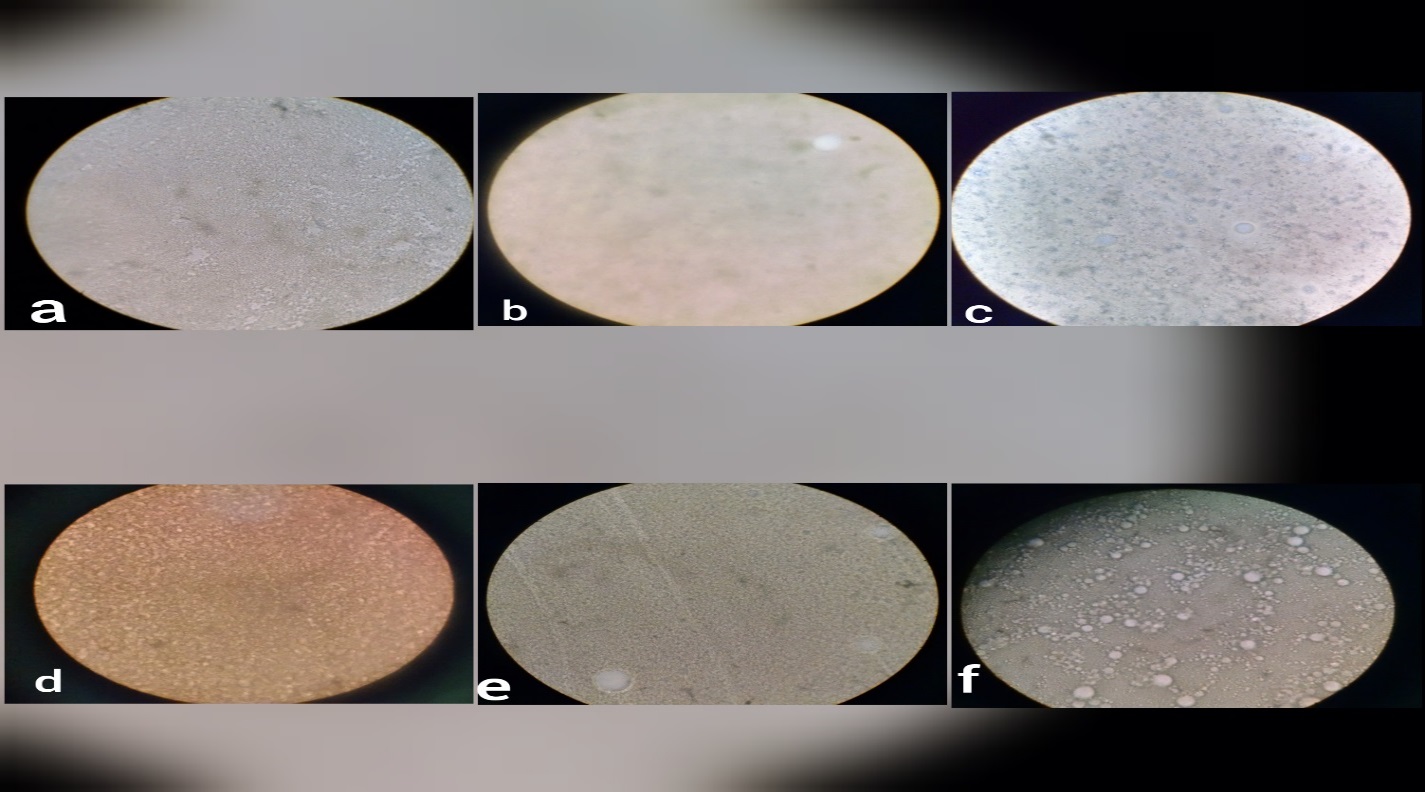
Otto Flu Plus vaccine was evaluated for change in physiochemical properties. This evaluation was done after storage at 4°C, 8°C, 12°C, 16°C and 20°C for 75, 90 and 120 days.
Otto Flu Plus vaccine stored at 4℃ for 75, 90 and 120 days showed mean viscosity values of 37.6±0.43, 37.6±0.23, and 37.5±0.31 mpa/sec respectively (Figure 2, Table 3). The vaccine stored at 8℃ showed mean viscosity values of 35.8±0.13, 35.5±0.35 and 35.6±0.31 mpa/sec (Figure 3, Table 3). At 12℃ showed mean viscosity values of 31.8±0.13, 31.5±0.85 and 31.0± 0.70 mpa/sec viscosity (Figure 4, Table 3). At 16℃ showed mean viscosity values of 28.3±0.78, 27.3±0.77 and 25.1±0.71 mpa/sec viscosity respectively (Figure 5, Table 3). And the vaccine stored at 20℃ for 75, 90 and 120 days showed mean viscosity values of 20.8±0.15, 17.6±0.18 and 15.5±0.32 mpa/sec viscosity respectively (Figure 6, Table 3).
Effect of accelerated temperature on pH at different time interval
Otto Flu Plus vaccine stored at 4℃ for 75, 90 and 120 days showed mean p H values of 6.68±0.10, 6.66±0.11 and 6.62±0.14 respectively (Figure 2, Table 3). The vaccine stored at 8℃ showed mean pH values of6.68±0.10, 6.66±0.11 and 6.62±0.14 (Figure 3, Table 3). At 12℃ showed mean pH values of 6.58±0.19, 6.54±0.11 and 6.54±0.13 (Figure 4, Table 3). At 16℃ showed mean pH values of 6.62±0.19, 6.50±0.70 and 6.46±0.54 (Figure 5, Table 3). Moreover, the vaccine stored at 20℃ for 75, 90 and 120 days showed mean p H values of 6.64±0.89, 6.50±0.12 and 6.48±0.13 respectively (Figure 6, Table 3).
Effect of accelerated temperature on Density at different time interval
Otto Flu Plus vaccine stored at 4℃ for 75, 90 and 120 days showed mean density values of 0.91±0.01, 0.91±0.01 and 0.90±0.01 respectively (Figure 2, Table 3). The vaccine stored at 8℃ showed mean density values of 0.91±0.00, 0.90±0.00 and 0.90±0.01 (Figure 3, Table 3). At 12℃ showed mean density values of 0.91±0.00, 0.90±0.00 and 0.90±0.01 (Figure 4, Table 3). At 16℃ for 75, 90 and 120 days showed mean density values of 0.89±0.01, 0.89±0.01 and 0.89±0.01 (Figure 5, Table 3). In addition, the vaccine stored at 20℃ for 75, 90 and 120 days showed mean density values of 0.89±0.01, 0.87±0.01 and 0.87±0.01 respectively (Figure 6,Table 3).
Effect of accelerated temperature on stability at different time interval
Otto Flu Plus vaccine stored at 4℃ for 75, 90 and 120 days showed mean stability values of 4.00±0.00, 4.00±0.00 and 4.00±0.00 respectively (Figure 2, Table 3). The vaccine stored at 8℃ showed mean stability values of 4.00±0.00, 4.00±0.00 and 4.00±0.00 (Figure 3, Table 3). At 12℃ showed mean stability values of 4.00±0.00, 4.00±0.00 and 3.20±1.09 (Figure 4, Table 3). At 16℃ showed mean stability values of 3.60±0.89, 3.20±1.09 and 2.80±1.09 (Figure 5, Table 3). And the vaccine stored at 20℃ for 75, 90 and 120 days showed mean stability values of 2.40±0.89, 2.40±0.89 and 2.00±0.00 respectively (Figure 6, Table 3)
Effect of accelerated temperature on particle distribution at different time interval
Otto Flu Plus vaccine stored at 4℃ for 75, 90 and 120 days showed mean particle distribution values of 6.00±0.00, 6.00±0.00 and 6.00±0.00 respectively (Figure 2, Table 3). The vaccine stored at 8℃ showed mean particle distribution values of 6.00±0.00, 6.00±0.00 and 6.00±0.00 (Figure 3, Table 3). At 12℃ showed mean particle distribution values of 6.00±0.00, 6.00±0.00 and 4.80±1.64 (Figure 4, Table 3). At 16℃ showed mean particle distribution values of 5.40±1.34, 4.80±1.64 and IV0±1.64 (Figure 5 Table 3). Moreover, the vaccine stored at 20℃ for 75, 90 and 120 days showed mean particle distribution values of 3.60±1.34, 3.60±1.34 and 3.00±0.00 respectively (Figure 6, Table 3).
Effect of accelerated temperature on serological potency of Otto Flu plus vaccine
Otto Flu Plus vaccine stored at 4℃ for 75, 90 and 120 days displayed 38.40±14.31, 38.40±14.31 and 38.40±14.31 mean anti-AIHI antibody titer 28 days’ post vaccination respectively. The vaccine stored at 8℃ displayed 44.80±17.52, 44.80±17.52 and 32.00±0.00 mean anti-AIHI antibody titer 28 days’ post vaccination. At 12℃ displayed 44.80±17.52, 44.80±17.52 and 32.00±0.01 mean anti-AIHI antibody titer 28 days’ post vaccination. At 16℃ displayed 30.40±21.46, 22.40±8.763and 17.60±8.76 mean anti-AIHI antibody titer 28 days’ post vaccination. And the vaccine stored at 20 ℃ for 75, 90 and 120 days displayed 30.40±21.47, 14.40±3.577and 12.80±4.38 mean anti-AIHI antibody titer 28 days’ post vaccination respectively. (Figure 7 Table 3)
Effect of storage conditions on Otto Flu Plus vaccine
Otto Flu Plus vaccine (FQ-235-OB) stored at 2℃ for 15 months showed milky white appearance with 36.46±1.10, 6.57±0.05, 0.89±0.001, 6.00±0.00, 6.00±0.00 and 1µm mean standard values of color, Viscosity, pH, density, stability, Particle distribution and particle size respectively (Figure 8 Table 4). The vaccine stored at 4℃ for 15 months showed milky white appearance with 37.61±0.04, 6.57±0.08, 0.90±0.00, 6.00±0.00, 6.00±0.00 and 1µm mean standard values of color, Viscosity, pH, density, stability, Particle distribution and particle size respectively. (Figure 9 Table 4). The vaccine stored at 8℃ for 15 months showed milky white appearance with 35.07±2.08, 6.46±0.01, 0.89±0.00, 4.76±0.09, 5.0±1.03 and 1µm mean standard values of color, Viscosity, pH, density, stability, Particle distribution and particle size respectively. (Figure 10 Table 4).
Effect of storage conditions on serological potency of Otto Flu Plus vaccine
Otto Flu Plus vaccine stored at 2℃ for 3, 6, 9, 12 and 15 months displayed 38.40±14.31, 38.40±14.31, 38.40±14.31, 38.40±14.30 and 38.40±1IV8 mean anti-AIHI antibody titer 28 days’ post vaccination respectively (Figure 10, Table 4). The vaccine stored at 4℃ displayed 34.65±4.3, 34.65±4.4, 34.65±4.3, 34.65±4.4 and 34.65±4.5 mean anti-AIHI antibody titer 28 days’ post vaccination (Figure 10, Table 4). At 8 ℃ displayed 32.00±1.54, 32.00±1.51, 32.00±1.50, 32.00±1.48 and 33.00±1.42 mean anti-AIHI antibody titer 28 days’ post vaccination respectively (Figure 11, Table 4).
Table 3
Effect of accelerated temperature on the stability of Otto Flu Plus vaccine at different time period
Table 4
Effect of real time study on the stability of the Otto Flu Plus vaccine at different time period
Discussion
Vaccination is considered as one of the strongest public health goal during the 20th century which reduces morbidity and mortality from a number of vaccine-preventable diseases. There are many types of routinely used vaccines including live attenuated, killed or inactivated, subunit and subunit-conjugated vaccine.5 Live attenuated organism often need complex formulations and careful handling as sometimes they are fragile organisms that need to be kept in a state in which they can replicate in order to stimulate immunity. So, they are lyophilized during manufacturing to maintain this viability. However, inactivated and subunit vaccines are more stable to the thermal stress and are able to stimulate the immune response efficiently. Inactivated vaccines are usually associated with adjuvants particularly mineral oil which encapsulates the antigen creating defense line against unfavorable conditions. However, Aluminium Based adjuvants are susceptible to freeze thawed damage.6
As these are biologicals and sensitive to both heat and cold environment, therefore need to be maintained within a properly organized range of temperature referred as “cold Chain”. Cold chain is also referred as “vaccine supply chain” or “immunization supply chain” that consists of a series of links that are designed to keep the vaccine in temperature ranges recommended by WHO, from the point of manufacture to the point of administration. Environmental stresses including inappropriate handling or reconstitution, excess agitation and exposure to light can result in loss of vaccine potency.6
Thermal environment has disruptive effect on protein structure of antigen by changing the order of amino acids.7 In the current study it was documented that viscosity of vaccine stored at 4°C and 8°C for 6 months did not show any significant difference (p>0.05). However, the viscosity of vaccine stored at 12°C, 16°C and 20°C for 6 months showed significant difference (p<0.05).
As the storage period increased to 15 months, it was observed that the viscosity and particle distribution of the vaccine stored at 2°C and 4°C for 15 months did not show any significant difference (p>0.05) as compare to the vaccine stored 8°C for 15 months (p<0.05). In contrast, Leonard stated in his study that the viscosities of oil emulsions decrease as temperature increase because high temperature makes the molecules of oil and emulsions to get higher energy from heat thus making them less viscous so the oil can flow easily.8 Goldwood & Deisberg reported that increase in temperature increase the mobility and settling rate of water droplet; making the interfacial films weakens and tension between the two phases. It reduces the viscosities of oil and increase in droplets collisions favoring coalescence. Thus, acceleration in process by heating helps to break the emulsion.9
It was found that particle distribution of vaccine stored at 4°C, 8°C and 12°C for 6 months did not show any significant difference (p>0.05). However the particle distribution of the vaccine stored at 16°C and 20°C for 6 months showed significant difference (p<0.05). While the vaccine stored at 2°C and 4°C for 15 months showed significant difference (p>0.05) as compare to vaccine stored at 8°C for 15 months (p<0.05). Allison observed the diameter of polymer particles is not so effected by increasing temperature however, the lower particle size distribution is due to rapid particle nucleation caused by high temperature.10 Clenet stated that similar droplet size in microscopy is a helpful factor in mai ntaining emulsion stability. Large particles contain less interfacial surface per unit volume than small droplets. An emulsion having a uniform size distribution is more stable than one with a wider size distribution with the same average particle size.11
While the pH, stability and density of vaccine stored at 2°C, 4°C and 8°C for 15 months did not showed any significant difference (p>0.05). Moreover, pH of the vaccine did not show any significant difference when stored at 4°C, 8°C, 12°C, 16°C and 20°C for 6 months. The density and stability of vaccine stored at 4°C, 8°C and 12°C for 6 months did not show any significant difference (p>0.05) as compare to the vaccine stored at 16°C and 20°C for 6 months (p<0.05).
The Anti-AIHI antibody titer of the vaccine stored at 2°C, 4°C and 8°C for 15 months did not show any significant difference (p>0.05). The similar results were recorded for the vaccines stored at 4°C, 8°C, 12°C and 16°C for 6 months. While there is a significant difference observed in Anti-AIHI antibody titer for the vaccine stored at 20° C storage temperature for 6 months (p<0.05). Whereas, Quan concluded that heating of an oil emulsion displayed a significant difference in titer at 18 weeks but the difference did not remain significant at 24 weeks.12
The results of the real time study concluded that Otto Flu Plus vaccine was stable at 2°C to 8°C when stored for 15 months. Whereas, in accelerated stability study the vaccine showed optimum result when stored at 4°C to 16°C for 6 months. There was least physiochemical deterioration at 16°C when stored for 6 monthss. Whereas, the significant deterioration in vaccine was recorded physically at temperature >12°C stored for 6 months. At 20°C the emulsion was broken and did not shown any significant protective titer in experimental birds.