Introduction
Evolution happens over time and more so in the bacterial world. These minute single celled organisms are evolving on a daily basis. The bacteria which were once considered too weak to cause harm, are improving their arsenal in proving to be the most troublesome pathogens of modern times. Resistance to antibiotics is the strongest weapon bacteria are using againstus and we do not have any promising antibiotics in the pipeline to combat antibiotic resistance. Clinically, the viable option is to use available antibiotics judiciously. We are in a phase where more and more resources are being spent for combating antimicrobial resistance. Newer techniques are being developed day by day to predict the pathogenic potential, and these require a large number of bacterial isolates from different clinical sources. Indian government has taken initiatives to assess the impact of antibiotic resistance and is planning a step wise approach to fight antimicrobial resistance. Currently we are in our initial steps trying to analyze the exact extent of damage caused by drug resistant microorganisms. The way forward to manage the menace of antibiotic resistance is to form a uniform, national, practically acceptable antibiotic policy. One of the main requirements for development and testing of newer methods as well as analysis of antibiotic sensitivity pattern is to collect large number of bacteria from different clinical samples. This calls for efficient bacterial storage so that large number of isolates can be tested at once. This will help develop concrete data on feasibility, pitfalls, variations in different isolates and possible modifications of the newer methods.
Bacteria have been stored in various forms. Currently most of the research labs as well as advance d laboratories use the lyophilisation or the freeze drying technique. This method is quite good in retaining the genetic as well structural stability of the bacterial cell. The storage space required for the resultant lyophilized culture is also considerably less. Various studies have proved that the revival of strains is much more compared to other methods. The lyophilized cultures have to be stored at -70°C to -80°C.1 Around 80% recovery of stored bacterial isolates has been documented. This method has been proved to be effective in long term storage as well. 50% of strains stored can be recovered by the end of 5 years. This method although superior has its own disadvantages. First and foremost is the cost involved in purchase of lyophilizer, consumables & deep freezer. Secondly the running cost which includes the increase in electricity bill, maintenance of the machinery and need for expensive expertise. These factors may not be acceptable to a small microbiology laboratory or a post graduate student working on short term project. We went back in time to understand what other methods have been used in the past to store the bacteria. Although many different methods have been tried, few methods were appropriate for short term storage of bacteria. We narrowed down to methods which have been used extensively in the past for short term storage of bacteria & Fungi. The criteria was to look for a cost effective, less labour intensive, technically simple that is devoid of any costly storage equipments.
Use of glycerol
Some studies suggest that bacteria were stored using this method for more than a decade. S. E. Hartsell in his study published in 1952 has reported that even fastidious organisms were stored in paraffin oil with modification for varying periods of 6-18 months.
Use of deionised water
This method has been used for storage of bacterial cultures for long periods of time.2 Study by CH Liao et al have reported viability of human pathogens upto 5 years.
Materials and Methods
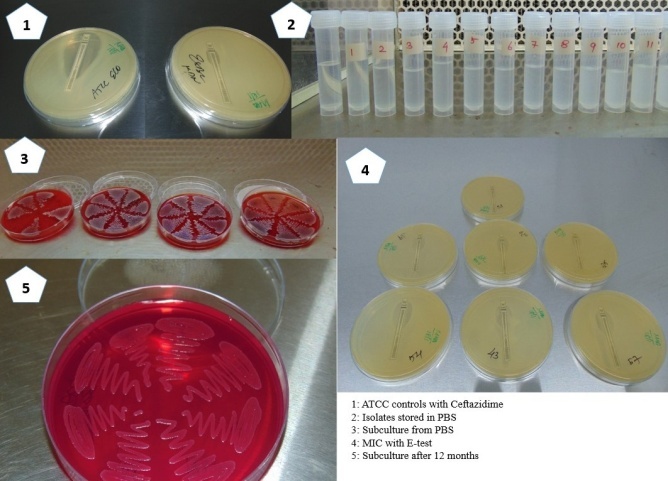
Bacterial isolates of Escherichia coli were obtained from microbiology lab of Azeezia Institute of Medical Sciences & Research from July 2016 to July 2017. The isolates were collected by judgmental sampling. A total of 1 35 isolates were collected during the study period. The department wise and gender wise distribution of isolates is presented in Table 1. The isolates were subcultured on 5% sheep blood agar and were incubated at 37°C for 18-24 hours.3,4,5 After incubation the isolates were analysed for florid growth. Standard biochemical tests were done to confirm the identity of isolates. These included Indole test, Methyl red test, Voges-proskauer test, Mannitol Motility, Citrate utilization test, Urease test and Triple sugar Iron agar.6,7,8,9 The confirmed isolates were then inoculated into autoclaved Phosphate Buffered Saline (PBS).2
The PBS was prepared in house and required chemicals were procured from Himedia lab supplies, Mumbai.10 These include Sodium Chloride (NaCl), Potassium Chloride (Kcl), Disodium Hydrogen Phosphate (Na2 HPO4) & Monopotassium Phosphate (KH2 PO4). 800ml of distilled water was taken in a conical flask. To this 8gms of NaCl, 0.2gms of Kcl, 1.44gms of Na2 HPO4 and 0.24gms of KH2 PO4 were added. Final pH of 7.4 was achieved by adding 200ml distilled water. After thoroughly dissolving all the ingredients and checking the pH the PBS was dispensed into 10ml screw capped plastic tubes (HIMEDIA). These screw capped vials were autoclaved at 121°C for 20mins at 15lbs pressure. Second autoclaving was done after 24 hours. One screw capped vial per batch was randomly selected and contents inoculated into Brain heart infusion broth (BHIB). This BHIB was incubated for 48hours to look for turbidity. Once the sterility of Phosphate Buffered Saline was confirmed the screw capped vials were used for isolate inoculation and storage.
Loopful of florid grown culture was taken from 5%Sheep blood agar and mixed with PBS in the screw capped vial. The vials were labelled and incubated at 37°C overnight to form a smooth emulsion.
Isolated colony was picked from the 5% Sheep blood agar plate and inoculated into Mueller Hinton Broth (MHB). MHB was incubated for two hours at 37°C. At the end of two hours the turbidity was compared with 0.5 McFarland standards. Immediately lawn culture was prepared on four Mueller Hinton agar plates. Three inoculated plates were used for Antibiotic Sensitivity testing used Modified Kirby Bauer method.10 The isolates were analysed for their sensitivity to Ampicillin(10μg), Gentamicin(10μg), Tobramycin (10μg), Amikacin (30μg), Ciprofloxacin(5μg) Levofloxacin(5μg) Cotrimoxazole (1.25/23.75μg) Amox- Clavulanic Acid(20/10μg) Piperacillin- Tazobactum (100/10μg) Norfloxacin (10μg) Amikacin(30μg) Ceftriaxone (30μg), Ceftazidime(30μg), Ceftazidime- Clavulanic Acid(30/10μg), Cefotaxime(30μg) Imipenam (10μg), Tetracycline(30μg) and Nitrofurantoin(300μg). The fourth plate was utilized for baseline value of Minimum Inhibitory Concentration (MIC) of Ceftazidime using the Epsilometer test (E-test). The E-Test strips were obtained from Himedia labs, Mumbai. Internal quality control was set up using E.coli strain ATCC 25922.11,12 The antibiogram of isolates is presented in Table 2.
The vials were stored at room temperature inside a thermocol box to avoid exposure to sunlight. The screw capped vials were analysed every 30 days. The analysis was to look for any kind of visible contamination & reduction in distilled water volume. Subcultures from distilled water were done to look for ability to grow, reduction in colony counts and for checking the purity of the isolate. At every six months interval, Antibiotic sensitivity testing and MIC using E-test were done and compared with baseline values.11
Results
During the study period a total of 135 samples were collected during the study period. Out of these 135 samples 6 isolates did not grow on subcultures.13 isolates got contaminated. 6 isolates were mixed with Pseudomonas and 4 of them with Aspergillus niger. These samples were discarded as contaminants. The remaining 119 isolates gave good growth till the end of 12 months study period. The Minimum Inhibitory Concentration (MIC) did not alter from the baseline MIC done at the time of recruiting the isolate for the study.
Table 1
| Department | Male | Female |
| Medicine | 21 | 36 |
| Urology | 10 | 2 |
| Casualty | 5 | 11 |
| Surgery | 3 | 6 |
| Paediatrics | 2 | 2 |
| Orthopaedics | 1 | 5 |
| Neurology | 1 | 1 |
| Obstetrics | 0 | 14 |
| Pulmonology | 0 | 1 |
| Others | 4 | 10 |
| Total | 47 | 88 |
E.coliisolate department & Gender wise distribution
Table 2
Antibiogram of E.coli isolates selected for the study
Discussion
Storing microorganisms for future study are valuable resources for scientific research in the field of Microbiology. These organisms help to bring in better understanding towards microbial diversity, evolution, study of evolution of antibiotic resistance patterns over time. The stored bacteria also help in epidemiological investigations and training in clinical microbiology. Preserved microorganisms serve as permanent records of microorganisms in terms of their phenotypic and genotypic profiles.14
There have been various methods described about effective storing of bacteria. This means that the bacteria have to be stored without contamination in a viable state and also preserving all phenotypic and genotypic characters. This can be achieved by storing the bacteria in maintenance medium. Maintenance medium has to have very minimal nutrients so as to reduce the rate of multiplication of bacteria. Increase in nutrients will lead to rapid rate of multiplication and eventually leading to frequent subcultures. Secondly every multiplication can have some mutations which need to be minimized to maintain the original Phenotypic & Genotypic charters of the original strain of bacteria. There have been many methods but in a resource poor country like India we need economical, less labor intensive and simple techniques.
The simplest technique for short term preservation of bacteria involves direct subcultures to fresh plates. The survival of the bacterial strain in assured but this process fails to address all other issues involved in storing the bacteria. The subcultures are usually done on plates and would require subcultures at least once in 7 days if stored in an incubator or room temperature. That will increase the cost of culture media used. This also increases the manpower need to subculture these samples and reduces the chances of storing larger number of strains. Even if done properly every new subculture increases the likelihood of mutations with undesirable changes in microorganisms. Part of these can be circumvented by inoculating the bacteria stored on agar slopes in a screw capped bottle or test tube, covered with a film and kept away from direct light. This method in our hands did not give satisfactory results. The chances of contamination were high. Secondly there is not set protocol for frequency of subcultures. Every bacterium requires different intervals of subculture and it has to be determined by trial and error method. Clinical stains of enterobacteriaceae family which have the ability of gas production also caused difficulties in this method of storage.
An alternative to capping bottles of using corked test tubes is to layer mineral oil on top of the specimen. Literature shows that most bacteria and fungi can be stored for nearly 2-3years. 5-10 colonies and inoculated on the agar slant and incubated. Once there is evidence of growth, 1-2cm layer of mineral oil is added. The agar should not be exposed to air. This method again has its own problems when tried. The main issues faced by us was that the revival rate of microorganisms covered with mineral oil was less compared to direct subcultures. Secondly the cleanup in the event of spills was a bit more difficult compared to routine protocol followed in a clinical microbiology laboratory. 14
It has been long documented that Fungi and pseudomonas sp can be stored in distilled water. These fungi and Pseudomonas sp have retained the ability to grow after several years of storage. Hence for this study the authors used the same principles to determine the ability of clinical strains of enterobacteriaceae survival using Phosphate Buffered Saline (PBS).
During our study it was decided to prepare PBS inhouse to reduce the cost involved. The PBS was prepared in house using the following protocol. Sodium Chloride (NaCl), Potassium Chloride (Kcl), Disodium Hydrogen Phosphate (Na2 HPO4) &Monopotassium Phosphate (KH2 PO4) were used. 800ml of distilled water was taken in a conical flask. To this 8gms of NaCl, 0.2gms of Kcl, 1.44gms of Na2 HPO4 and 0.24gms of KH2 PO4 were added. Final pH of 7.4 was achieved by adding 200ml distilled water. After thoroughly dissolving all the ingredients and checking the pH the PBS was dispensed into 10ml screw capped plastic tubes. These screw capped vials were autoclaved at 121°C for 20mins at 15lbs pressure. Second autoclaving was done after 24hours. One screw capped vial per batch was randomly selected and contents inoculated into Brain heart infusion broth (BHIB). This BHIB was incubated for 48hours to look for turbidity. Once the sterility of Phosphate Buffered Saline was confirmed the screw capped vials were used for isolate inoculation and storage. The bacterial strains were initially grown on 5% Sheep Blood agar. The same isolates were evaluated for base line antibiotic sensitivity as well as MIC to ceftazidime. The vials were stored away from direct sunlight, at room temperature in a thermocole box. Subcultures at the interval of 6 months showed good revival rates, lesser contamination rates as well as no change in the antibiotic sensitivity pattern or Minimum Inhibitory Concentration to Ceftazidime
This method is Simple, cost effective, does not require sophisticated machinery or technical training. This method is suitable for use even in smaller laboratories with minimal resources. Provided the fact that the bacterial strains can be stored in large numbers with meagre investment the data can be used to analyze the pattern of evolution of antibiotic resistance in bacteria. As it is common knowledge that there is paucity of newer antibiotics coming to help treat life threating infections, the stored bacteria can be used effectively to predict and analyze the effect of the antibiotics in clinical isolates. India is currently facing the epidemic of antibiotic resistance among gram negative bacteria. Currently the government of India has made control of antibiotic resistance its main priority. As per the suggestions of the Chennai declaration every patient care unit big or small is expected to develop its own antibiotic policy.15 For development of antibiotic policy we first need to have data on the antibiogram of isolates received in the patient care unit. For small clinics or private nursing homes it might be difficult to test for all drugs and form an antibiogram. In such cases the isolates can be stored and minimum inhibitory concentrations (MIC) can be worked out at a later date. This can in turn result even in the smaller health care units having a robust antibiotic policy.
Conclusion
The short term trial study shows that this is inexpensive, technically simple method of bacterial conservation and is ideal for short term storage. The problem of contamination of isolates was minimal and can be prevented by triplicate inoculation of vials. The revival rate of bacterial isolates was very good. The MIC had not changed when compared to baseline. So this method proves to be quite useful for Antibiotic resistance studies as well. The way forward is to evaluate the maximum amount of time E.coli can stay alive in PBS without any changes in the MIC. This method will prove to be useful for young researchers, Post graduate students and even Microbiology labs with minimal facilities.