Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) and glycopeptide-resistant Enterococci are frequent pathogens in hospitals, requiring the need for new antibacterial drugs that lack cross-resistance to existing pathogens.1 Linezolid is the first oxazolidinone to be licensed for clinical use which exerts its antibacterial action by inhibiting the formation of the 70S initiation complex. 2 This in turn prevents the translation and replication of bacterial proteins. Linezolid provides high rates of clinical cure and microbiological clearance in complicated infections due to Enteroccus spp., including Vancomycin resistant Enterococcus and other Gram positive bacteria like Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae.2, 3 Linezolid is a broad spectrum antibiotic, used as a first line antibiotic for infections like hospital-acquired pneumonia caused by Staphylococcus aureus, uncomplicated SSSIs caused by methicillin-susceptible S. aureus(MSSA) or Streptococcus pyogenes, complicated skin and skin structure infections (SSSIs) and sepsis caused by Methicillin-resistant S. aureus (MRSA) and community-acquired pneumonia caused by Streptococcus pneumoniae. 4, 5 Linezolid is also used as a third-line anti-tubercular drug WHO group 5.6
Linezolid has 100% bioavailability and extensive volume of distribution after oral administration. It is excreted either by non-renal clearance after being metabolized into inactive metabolites (65%) or being unchanged by renal clearance. 2 In the patients with renal insufficiency, plasma concentration of linezolid is not affected, whereas vancomycin excretion is impaired causing higher systemic toxicity. Hence in renal failure cases it is the preferred drug over vancomycin. 7 It is frequently administered as an intervention in sepsis and septic shock patients as a broad spectrum coverage for Gram positive pathogens. 8, 9 But extensive use of linezolid has led to evolvement f linezolid resistant Enterococci which is of concern to the clinicians.
Clinical resistance to linezolid is found to be associated with a G2576T mutation in domain V of 23 S ribosomal ribonucleic acid (rRNA) genes of Enterococcus and level of linezolid resistance is directly related to the number of 23S rRNA genes containing this mutation. Both laboratory and clinical strains of E. faecium with linezolid minimum inhibitory concentrations (MICs) of 4 μg/mL have been shown to carry the G2576T mutation. 10 Other factors such as indwelling intravascular devices, under dosage prescription of the antibiotic, immunosuppression after transplantation and long courses of linezolid therapy (20-40 days) as in infective endocarditis and bone and joint infections are the other predisposing factors for the development of linezolid resistance. 11, 12 Accurate detection of low level of linezolid resistance due to G2576T mutation in one or two genes is necessary by susceptibility testing methods, as this can prevent higher levels of resistance associated with extensive use of the antibiotic. 13
Suggested modalities of acquisition of linezolid-resistant and vancomycin-resistant Enterococcus faecium (LR VREF) are: (i) de novo selection of resistant mutants in colonizing/infecting VREF (patients who carried genetically unrelated strains), (ii) patient to patient spread (patients who carried genetically related strains) and (iii) emergence of LR mutants from linezolid intermediate vancomycin resistant Enterococci (LI VRE) during the linezolid therapy. 14 Old reports of nosocomial transmission of LR VREF from a linezolid-treated patient to several untreated patients causing asymptomatic colonization has been known.15
Here, we report a case of linezolid resistant Enterococcus faecium in a case of sepsis admitted to a tertiary care hospital in South India. The isolate had a peculiar susceptibility pattern being sensitive to vancomycin, but highly resistant to linezolid with an MIC of 1024 μg/mL. Our case was also exceptional in a way that, the patient did not have any previous exposure to linezolid. There are no reports of VSLR(Vancomycin sensitive Linezolid resistant)cases from South India. Ours will be the first report of such a case from South India.
Case
A 32-year-old female, known case of hypothyroidism since 8 years, presented to our hospital with chief complains of right sided abdominal pain and high grade fever associated with chill and rigor. Patient was apparently alright 3 months back, then she developed intermittent low- grade fever and right hypochondriac pain along with the yellowish discoloration of sclera and clay colored stools. With these complains she presented to our hospital on 5th February, 2019, where she was diagnosed with choledocholithiasis and cholangitis. She had to undergo upper gastrointestinal surgery with ERCP (Endoscopic retrograde cholangio-pancreatography) and sphincterotomy on 15th February, 2019, after which she improved symptomatically. On follow up Contrast enhanced CT scan, she had multiple micro-abscesses in liver. She was discharged on post-operative day 5 and advised to review after 2 weeks. But 4 days after being discharged patient continued to have low grade fever, jaundice, clay colored stools, abdominal pain, tachypnea and palpitation for which she was re-admitted to the emergency medical services of our hospital and found to have tachycardia following which she went into ventricular fibrillation, followed by cardiac arrest. The patient was revived by putting her on injection intravenous noradrenaline and was intubated and put on ventilator support. Vitals were monitored continuously. Episode of supraventricular tachycardia was reversed by injection adenosine.
Computerized tomographic scan done on day 2 of re-admission showed normal size gall bladder and liver but multiple micro-abscesses in the liver parenchyma. Chest X-ray showed right lower lobe consolidation. Patient was managed symptomatically and then shifted to critical care unit for further management. At the time of admission to CCU, the patient neurological score was 8/15 as per Glasgow Coma scale. The pulse was 88/min, blood pressure-140/80 mm Hg, respiratory rate of 16/min and was irregular with bilateral vesicular breath sounds. The random blood glucose was 167 mg/dL. The other blood investigations were as follows: Total leukocyte count-12,700/mm3 with neutrophil predominance(88%), serum alanine aminotransferase (ALT) -52 IU/mL, serum aspartate aminotransferase (AST) -123 IU/mL, alkaline phosphatase-221 IU/L, total protein-5.7 g/dL, albumin-2.5 g/dL, urea-52 mg/dL, creatinine-1.27 mg/dL, total bilirubin-4.82 mg/dL, conjugated bilirubin 2.85mg/dL. Procalcitonin value on day 2 of admission was 68.17 suggestive of septic shock. Chest X-ray done on day 2 showed right lower lobe consolidations.
The patient was diagnosed with septic shock, based on symptoms and procalcitonin values. After stabilizing the vitals, the patient was started on piperacillin + tazobactam and linezolid empirically before which one set of blood culture bottle was sent from peripheral line and central line. Regular investigations such as regular complete blood count, alternate day renal function test, liver function test and procalcitonin level were sent for daily evaluation of patient status. Blood culture was sent in BACT/ALERT 3D bottles which were loaded into BACT/ALERT VIRTUO instrument, followed by performance of antibiotic susceptibility testing by VITEK 2 instrument. Blood culture from peripheral line grew Escherichia coli sensitive to only Amikacin and colistin and Enterococcus faecium sensitive to vancomycin alone and resistant to ampicillin, tetracycline, high level gentamicin and linezolid with a time to positivity of 28 hours. Blood culture sent from the central line also grew Enterococcus faecium of same sensitivity pattern with a time to positivity of 30 hours. Patient’s tracheal aspirate culture also grew the Enterococcus faecium with same antibiotic susceptibility pattern, but the urine culture was sterile. The patient was started on colistin and vancomycin based on antibiotic susceptibility testing performed by VITEK 2 AST cards.
On day 6 of treatment, patient had low grade fever with stable vitals on ventilator. The routine blood investigations showed total leukocyte count of 9290/mm3 with neutrophil predominance (71.4%) and renal parameters were normal. But total bilirubin was 7.64 mg/dl (direct bilirubin-4.18 mg/dl), AST was 50 and ALT was 22 IU/lit. Repeat culture and sensitivity testing of urine sample from catheter was sterile and from blood the same organisms grew. Hence the same antibiotics were continued and the general condition of the patient improved, with total leukocyte counts dropped to normal level and procalcitonin value of 3.62 ng/ml on day 8, in comparison to initial procalcitonin value which was 25 ng/ml.
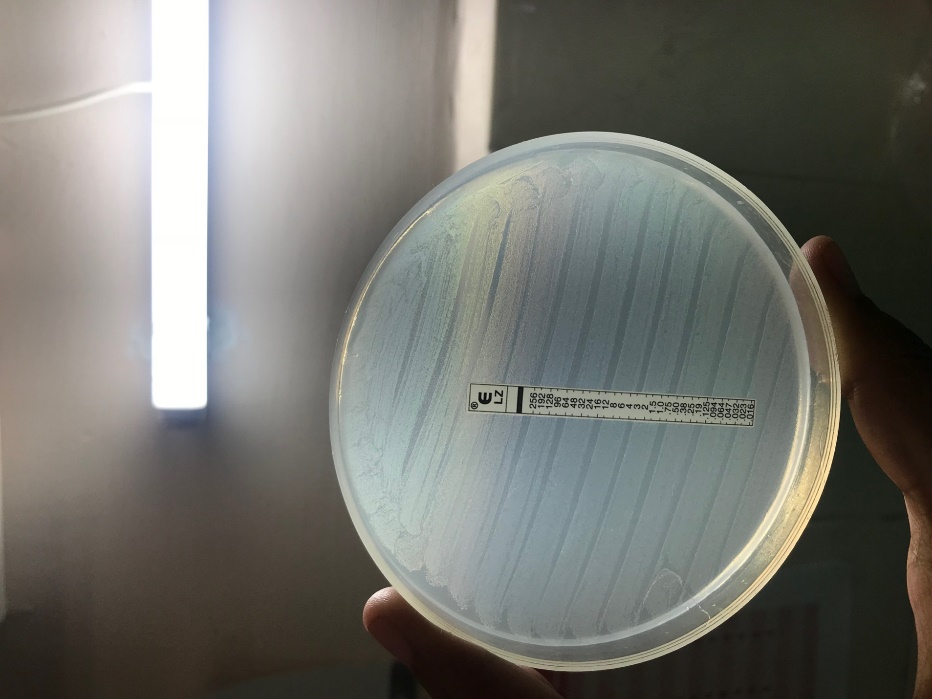
E. faecium isolate was identified by standard laboratory procedures and MALDI-TOF MS. The antibiotic susceptibility testing of the isolate was performed by Kirby Bauer disc diffusion method with a 0.5 McFarland standard inoculum as per Clinical and Laboratory Standards Institute (CLSI M100) guidelines,12 which showed the organism to be sensitive to vancomycin, but the isolate was resistant to linezolid, ampicillin and high level gentamicin. Disk diffusion testing for linezolid was performed using 30-μg linezolid disks (BBL, Becton Dickinson) showed no zone around the disc. Linezolid resistance was further confirmed with linezolid E-strips (Hi Media laboratories Mumbai) with a concentration gradient corresponding to 0.016-256 μg/mL on Mueller-Hinton agar as per manufacturer’s guidelines using 0.5 McFarland standard inoculum. After 24 hours of incubation at 37 °C, the isolate did not show any zone of inhibition, indicating that the MIC of the isolate was more than 256 μg/mL [Figure 1]. Further confirmation of the result was done by automated MIC determination method by VITEK 2 system using VITEK 2- AST P 628 card (BioMérieux) according to the manufacturers’ instructions. The categorical interpretation of results was based on recent CLSI guidelines.16
Patient was afebrile on day 9 and 10. Procalcitonin value on day 10 was 1.53 ng/ml. Repeat blood culture sent from both central and peripheral line sent on day 10 were sterile following which the patient’s central line was removed. Patient was extubated on day 11. She was discharged with an advice of continuation of antibiotics for 5 more days and to follow up after 2 weeks.
The swabs from the other patients in the same ICU as well as from the ICU floor, beds and ventilator were sent for surveillance purpose, as the isolated organism had a potential for nosocomial spread through hands of health care workers. But Enterococcus species was not isolated from any of the samples, implying that it was not a nosocomial infection.
Discussion
Enterococcal infections are common in liver transplantation and hepato-pancreatico-biliary (HPB) surgery. Linezolid is frequently used to treat vancomycin-resistant Enterococcus (VRE) as well as vancomycin-sensitive Enterococcus(VSE) and Methicillin resistant Staphylococcus aureus (MRSA) infections in hospital community.17 Here we report a case of linezolid-resistant Enterococcus faecium from south India with very high linezoild MIC (>256μg/mL) but vancomycin MIC of 2 μg/mL, from a patient having no history of previous linezolid medication. Only one such case has been reported by Simit Kumar et al in 2014 in West Bengal.14 Ours is the second report of such an isolate from India and first such case from South India.
Such LR VREF(Linezolid Resistant Vancomycin Resistant Enterococcus faecium) and LR VSEF Linezolid Resistant Vancomycin Sensitive Enterococcus faecium) are of concern, if isolated from a hospital set-up, because of the possibility of nosocomial transmission via hands of the health-care workers or through the contaminated fomites. Hence infection control measures should be taken focusing on preventing infections and interrupting patient-to-patient transmission of these drug-resistant pathogens. 18 There are reports of increasing incidence of linezolid resistance in the same ICU of the hospital after isolation of linezolid intermediate or resistant strains. 3 In our case the surveillance culture from the rectal swab of the patient as well as from the patients admitted in same and different ICUs throughout the hospital were negative for linezolid resistant Enterococci.
Various methods of detection of linezolid resistance have been documented, such as Kirby-Bauer disc diffusion method, automated MIC testing by Vitek 2 , E-test method using linezolid E-strips containing linezolid of range 0.016-256 mg/L concentration and gold standard being the agar dilution method. Comparison all these methods with presence or absence of the G2576T mutation have been studied and documented. Vitek 2 (BioMérieux) MIC determination method using AST P-628 card showed poor correlation with G2576T mutation, hence lacked validation in appropriate identification of LR strains of Enterococcus. The disk diffusion testing was more specific for detection of fully susceptible strains without G2576T mutation, but was less sensitive than dilution methods for detection of decreased linezolid susceptibility due to G2576T mutation. 10
Whereas E-test result interpretation considering 80% growth inhibition at end points by visual examination was more sensitive than the above two methods, but subjected to observational variability. 10 The best correlation was shown by agar and broth dilution methods which was in full concordance with polymerase chain reaction for detection of G2576T mutation.5 We, in our case, have employed the disc diffusion method for initial detection of sensitivity to vancomycin alone and resistance to ampicillin, high level gentamicin and linezolid. Hence this peculiar report was followed by MIC determination by Vitek 2 (BioMérieux) using AST-P628 cards, which showed the same sensitivity pattern as that of disc diffusion method. Most of the previous reported strains of LREF were also resistant to vancomycin and teicoplanin, to ampicillin and to high concentrations of gentamicin, but our strain showed a peculiar pattern of vancomycin sensitivity and though resistant to others.14, 15 Linezolid resistance was further confirmed by the E-test method.
The alarming finding of rapid emergence of resistance to linezolid in E. faecium isolates during the linezolid therapy contradicts previous reports indicating that such resistance arises only after prolonged therapy with this antibiotic.15
Linezolid is an orally effective having a better oral bioavailability and it does not require renal dose adjustment. Hence it has been used inadvertently in treating MRSA (Methicillin Resistant Staphylococcus aureus), VRE(Vancomycin Resistant Enterococci) and even VSE(Vancomycin Sensitive Enterococci) strains in hospital settings as well as outside. 2 This has led to increasing incidence of linezolid resistance, especially in the hospital set-ups.
An additional concern is the risk of nosocomial spread of organism. There is a little experience with these infections and specific infection control measures have yet to be formulated. We suggest that the issues that the emergence of resistance to linezolid should be considered as a warning signal, especially considering the fewer armamentarium of antibiotics, effective against enterococci, which is one of the leading causes of nosocomial infections. The clinicians should also be aware that the indiscriminate prescription of this oral drug in the outdoor as well as indoor patients may lead to the emergence of linezolid intermediate and linezolid resistant cases of Enterococcus in the hospital or even the community.