Introduction
Herpes simplex virus (HSV) is a double stranded DNA virus belonging to family Herpesviridae. It is classified into HSV-1 and HSV-2 subtypes. HSV-1 infection shows primary symptoms of fever, headache and in severe form leads to meningitis, encephalitis and keratoconjunctivitis. On the other hand, HSV-2 causes genital herpes, contributing to the risk of HIV infection through sexual route (Whitley 1990; Brugha et al. 1997; Severson and Tyring 1999).1, 2, 3 HSV-2 is sexually transmitted virus and infect 500 million people worldwide and 23 million new infections are reported annually (Looker et al. 2008).4 After the primary infection, HSV tends to persists in the neuron of the ganglia and get reactivated from its latent state during deficiency of immunity, causing serious systemic illnesses (Whitley 1990).1 Since virus is an intracellular parasite in neuronal ganglia, it is difficult to completely eliminate it (Brugha et al. 1997; Severson and Tyring 1999; Looker et al. 2008).2, 3, 4 Nucleoside derivatives such as acyclovir, valaciclovir, famciclovir and cidafovir have been widely used for the treatment of HSV infection (Cassady and Whitley 1997).5 In addition to high cost, treatment with acyclovir and other related drugs is also associated with the emergence of drug resistant strains (Englund et al. 1990).6 Long term treatment and mutations are major obstacles in the treatment of immunocompromised patients, who are also infected with other opportunistic infections (Seang et al., 2014).7
Natural products form medicinal plants have been an important source of new biologically effective compounds exhibiting different modes of action against viral infection (De Clercq 2000; Newman et al. 2000).8, 9 The present study was focused on to evaluate the anti-HSV-2 activity of two important traditionally used medicinal plants, Lepidagathis trinervis Nees (Acanthaceae) is a prostrate to sub-erect, up to 30 cm tall undershrub and its ashes were used to cure eczema (Singh et al., 2002).10 L. trinervis shows anticancer activity against L1210 lymphoid leukemia and hypotensive effect (Iwu, 1993).11 Antioxidant and antibacterial properties were also prove significant in this plant (Jain et al., 2010; 2012).12, 13 Immunosuppressive tryptophan-derived alkaloid was isolated from L. cristata (Ravikanth et al., 2001).14
Sericostoma pauciflorum Stocks ex Wight (Boraginaceae) is a short straggling undershrub growing widely throughout sea coast of Saurashtra and Maharashtra. Plant is generally used in dehydration and acidity. It is used in making an important drug in Ayurveda named “Krishnavalli”. It is anticancer, antidiabetic, used in dehydration, acidity, and health promoting drug (as described in “Nighantu Ratnakar”). Phytochemically fernane, hopane and other type of triterpenoids were isolated from the plant (Afza et al., 1992; Ayatollahi et al., 1992).15, 16 Recent observations from our laboratory have documented anti-HIV activity, which is primarily mediated by inhibition of HIV-1 protease and Tat-long terminal repeat transactivation (unpublished observations).
Keeping in view that both plants possess effective anti-HIV properties, the 50% ethanolic and aqueous extracts prepared from whole plant of S. pauciflorum and leaves of L. trinervis were evaluated for anti-HSV-2 activity using in vitro plaque reduction assay. Subsequently, these extracts were also evaluated using different assay formats to determine at what stages of virus infection these may be acting.
Materials and Methods
Collection of plant materials and preparation of extracts
S. pauciflorum whole plant and L. trinervis leaves were collected from University campus, shed dried, grinded and strained through 30 mesh (0.5 mm). The botanical identity was confirmed by Herbarium, Department of Botany, University of Rajasthan, Jaipur. Voucher specimens of these plants, S. pauciflorum (110) and L. trinervis (64) has been deposited at the Herbarium and laboratory for further reference.
To prepare aqueous and 50% ethanolic extract finely grounded plant material (100 gm) was treated with 500 ml MilliQ water (60-75ºC for 6-8 h) and with ethanol: water (1:1 v/v; 500 ml at 25-30ºC overnight) respectively. The procedure was repeated three more times. The resultants were filtered through Whatman filter paper number 1, vacuum dried and lyophilized powder stored at -20ºC
Viruses and cell lines
Vero cells (a cell line from a normal adult African green monkey’s kidney) were obtained from National Centre for Cell Science, Pune and grown on Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich Inc., St. Louis, MO, USA) supplemented with 10% Fetal bovine serum (FBS) and the antibiotic-antimycotic solution [penstrep - ampho sol; Penicillin (10000 units ml-1), Streptomycin (10 µg ml-1) and Amphotericin B (0.025 mg ml-1) Biological Industries, Kibbutz Beit Haemek, Israel]. The cells were maintained at 37oC in a humidified atmosphere of 5% CO2. Herpes simplex virus type 2 (HSV-2) G strain, ATCC VR-734 (obtained from ATCC, Rockville, USA), was propagated in Vero cells. After three cycles of freeze/thaw, the supernatant was collected. The supernatant at increasing dilution was used to infect the Vero cells and plaque forming units (PFU) were determined as described previously (Burleson et al. 1992)17 and virus stock stored in aliquots at -80°C until use.
Acyclovir (commercial name acycloguanosine) was purchased from Sigma-Aldrich Inc. It was dissolved in dimethyl sulphoxide (DMSO) and then diluted with sterile deionized distilled water before use. The final concentration of DMSO was <0.1%.
Cytotoxicity assay
Cytotoxicity of the plant extracts on Vero cells was assessed using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich Inc.] assay (Mosmann 1983).18 Briefly, Vero cells (15×103/well 100 μl-1) were seeded in a 96-well culture plate (Greiner Bio-One, GmbH, Frickenhausen, Germany) and grown overnight at 37°C in a humidified atmosphere of 5% CO2. Next day, culture medium with increasing concentrations of various extracts was added in duplicate and further incubated for 72 h. Appropriate solvents, used to prepare various extracts were included as negative controls. After incubation, 10 μl of MTT reagent (5 mg ml-1) was added per well, incubated at 37°C for 3 h followed by addition of MTT solvent (100 μl well-1; 20% SDS and 50% dimethyl formamide in 50 mM PBS). The absorbance (OD) was read at 570 nm with reference filter at 690 nm. Percent cell viability was calculated by dividing the absorbance observed in experimental group by the absorbance in untreated group multiplied by 100. Values are expressed as CC50 that represent the concentration of the extract showing 50% cell viability.
Plaque reduction assay
This assay was carried out using standard protocol as described previously (Shigeta et al. 1992).19 Vero cells were grown in 24-well plate (FALCON 3046; 8×104 cells well-1) and incubated for 24 h at 37°C. After incubation, medium was removed, cells were inoculated with 300 µl of virus suspension (200 PFU per well) and the plates were further incubated for 1 h at 37°C under humidified atmosphere of 5% CO2. After virus adsorption, excess viral suspension was removed by washing twice the cells with fresh medium and cells were overlaid with an agar overlay medium containing varying concentrations of the test compounds (6.25, 12.5, 25 and 50 µg ml-1). Agar overlay with solvent used as negative control whereas the well containing Acyclovir (10 μM) was used as positive control. Each plant extract/compound was tested in duplicate in two separate experiments. After 72 h incubation, 24 well test plates were fixed with 10% formalin (in 50 mM PBS), followed by staining with 0.2% crystal violet. The number of plaques was counted. The average plaque count from duplicate wells at each concentration of test compound was plotted against the average plaque count of three untreated virus infected wells. The concentration required to reduce the plaque number by 50% (IC50) was calculated from the mean dose response curves of at least two independent plaque reduction assays.
Attachment assay
Vero cells (80,000 cells well-1) growing in 24 well plates were pre-chilled at 4°C for 15 min before experiment. Cells were infected with HSV-2 (200 PFU) in serum-free DMEM for definite time periods of 0.5, 1, 1.5 and 2.0 h at 4°C in the presence or absence of serial dilutions of plant extracts (6.25, 12.5, 25 and 50 µg ml-1). Unadsorbed virus was then removed by washing with sterile 50 mM PBS pH 7.4, cells overlaid with medium and nutrient agar. After 72 h cells were fixed, stained and processed for counting the plaques as described above (Piret et al. 2002).20
Penetration assay
In penetration assays, HSV-2 (200 PFU) was adsorbed on Vero cells growing on 24 well plates for 2 h at 4°C. The medium was replaced with pre-warmed fresh medium containing plant extracts (6.25, 12.5, 25 and 50 μg ml-1) and the temperature was increased to 37°C to maximize virus penetration. Cells were incubation for 0.5, 1, 1.5 and 2 h with plant extract. After incubation, cells were treated for 1 min with 50 mM PBS (pH 3.0) to inactivate the unpenetrated virus. After washing three times with serum-free medium, cells were overlaid with DMEM-0.5% agarose to quantitate surviving virus versus time of plant extracts treatment by counting plaque formation as described above (Piret et al. 2002) 20.
Post-attachment virus neutralization assay
Briefly, prechilled cells (2 h at 4°C) were incubated with HSV-2 (200 PFU) at 4°C for 2 h to allow stable attachment of virus without fusion with cell membranes. Medium was removed and plant extracts in DMEM were added in different concentration (6.25, 12.5, 25 and 50 μg ml-1) and incubated further for 2 h at 4°C. Cells were then washed and overlaid with DMEM plus 0.5% agarose and incubated at 37°C for 24, 48 and 72 h in humidified atmosphere of 5% CO2. For cell control, HSV-2 virus was incubated with serial dilutions of extracts for 2 h at 4°C prior to adsorption to cells (pre-attachment neutralization). Cells were treated as described earlier for plaque number reduction assay (Piret et al. 2002).20
Results and Discussion
Cellular toxicity
The inhibitory activity of the plant extracts against HSV-2 infection may be as a result of their toxic effect and therefore might result in an erroneous conclusion. Hence to exclude the non-specific antiviral effect, the toxicity of plant extracts on HSV-2 target Vero cells was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Mosmann 1983).18 The CC50 values of 50% ethanolic and aqueous extracts prepared from S. pauciflorum and L. trinervis ranged from 301.8 to 436.8 μg ml-1 (Table 1).
Antiviral activity
The activity of aqueous and 50% ethanolic extracts prepared from the leaves of L. trinervis and whole plant of S. pauciflorum have been evaluated for their anti HSV-2 activity using plaque reduction assay (Shigeta et al. 1992).19 These extracts have been tested at 4 concentrations ranging from 6.25 to 50 µg ml-1, whereas their toxicity analysis was done from 62.5 to 500 µg ml-1. The results are summarized in Table 1. Among the tested extracts, L. trinervis 50% ethanolic extract demonstrated lower IC50 value (6.9 µg ml-1), whereas both 50% ethanolic and aqueous extracts of S. pauciflorum showed activity with IC50 value of 11.4 µg ml-1 and 13.8 µg ml-1, respectively (Table 1). Acyclovir was used as standard reference drug and at 10 µM was completely inhibiting plaque formation by HSV-2 virus. The therapeutic index (TI) of a drug is the ratio between the toxic and the therapeutic dose and is used as a measure of its relative safety. The 50% ethanolic leaves extract of L. trinervis was more potent against HSV-2 as TI of the extract was 44.0 (Table 1).
Table 1
In vitro cytotoxicity and anti-HSV-2 activity of the extracts derived from stem bark of S. pauciflorum and leaves of L. trinervis
|
Plants tested |
Extracts used |
CC50 (μg/ml)* |
IC50 (μg/ml)* |
TI |
|
S. pauciflorum |
50% ethanol |
319.9 |
11.4 |
28.0 |
|
Aqueous |
334.2 |
13.8 |
24.3 |
|
|
L. trinervis |
50% ethanol |
301.8 |
6.9 |
44.0 |
|
Aqueous |
436.8 |
21.0 |
20.8 |
[i] *CC50: The cytotoxic concentration of the extracts that caused the reduction of viable cells by 50%. All data presented are averages reading of two independent experiment performed in duplicate.
Attachment assay
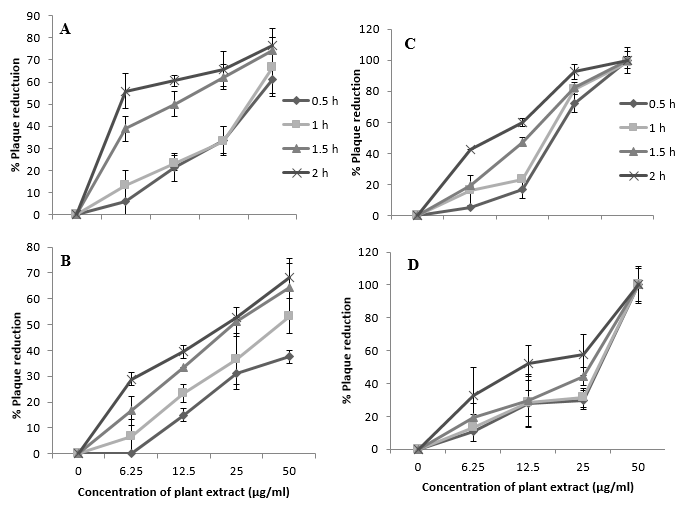
According to the results of time course studies, virus attachment was significantly inhibited both the extracts of S. pauciflorum (IC50 values of 26.8 to 7.6 µg ml-1 by 50% ethanolic and 69.2 to 20.7 µg ml-1 by aqueous extracts, respectively; Figure 1). Further, both 50% ethanolic and aqueous extracts from L. trinervis also inhibited the attachment of HSV-2 to Vero cells in a dose dependent manner. After exposure of Vero cells with L. trinervis extracts (50 µg ml-1) for either 1.5 or 2.0 h at 4oC, ≥ 90% inhibition in the attachment of HSV-2 was observed. In contrast, acyclovir which inhibits HSV replication, failed to show any significant inhibition in the virus attachment to Vero cells when tested up to 10 µM (data not shown).
Anti-HSV activity of natural products from other plants has been shown previously (Nawawi et al. 1999, 1999, 2001).21, 22, 23 Antimicrobial, antioxidant activities were evaluated and α Amyrin, β Amyrin, β-Sitosterol, friedelin and caffeic acid were isolated form S. pauciflorum (Pancholi, 2022).24 The interaction between legend and receptor is a complex step involved in virus infectivity, and our results showed that both 50% ethanolic and aqueous extracts effectively inhibit attachment of the virus particle.
Figure 1
Effectsof S.pauciflorum and L. trinervis 50% ethanolic (A and C) and aqueous extracts (B and D respectively) on attachment assay of HSV-2: Prechilled Vero cells were infected with 200 PFU of HSV-2 in presence or absence of serial dilution of the extracts at different time period at 4oC. S. pauciflorum 50% ethanolic extract showed 76.32% inhibition at 50 µg ml-1 concentration. On the other hand aqueous extract demonstrated 68.42% inhibition at 50 µg ml1 concentration with respect to cell control. L. trinervis both extract showed ≥ 90% inhibition at 50 µg ml-1 concentration. The results showed that HSV attachment was significantly inhibited at concentration and time dependent (IC50 values 7.56 and 11.74µgml-1 in 50%ethanolic and aqueous extract respectively at 50 µg ml-1 concentration).
Penetration assay
In penetration assay, plant extracts were added to the Vero cells after the initial binding of HSV-2 at 4oC. Both 50% ethanolic and aqueous extracts of S. pauciflorum failed to inhibit penetration in in vitro assay system (data not shown). Previously Cordia americana of family Boraginaceae were prove effective against HSV-2 viral penetration (Costa et al. 2012)25 but due to antagonistic effects of some other complex phytochemicals, S. pauciflorum plant extracts (50% ethanol as well as aqueous) does not show inhibitory activity on virus penetration. According to previous studies, caffeic acid derivative reduce penetration of HSV via disturbing viral glycoproteins (Ikeda et al. 2011).26
L. trinervis extracts showed activity in dose and time dependent manner (Figure 3). After virus adsorption, Vero cells treated with 50% ethanolic extract for 2 h at 37oC showed an IC50 value of 12.6 µg ml-1, which was more effective then aqueous extract (IC50 value of 33.7 µg ml-1; Figure 3). Clinacanthus nutans and C. siamensis from family Acanthaceae show potent inhibition of HSV. Monoglycosyl diglycerides and digalactosyl diglyceride were the active ingredients that possess the anti-viral potentials (Janwitayanuchit et al., 2003; Kunsorn et al., 2016).27, 28 This may be one of the possible reasons for the efficacy of both the extracts of L. trinervis in inhibition of HSV virus penetration.
Figure 2
Effects of L. trinervis 50% ethanolic (A) and aqueous extracts (B) on penetration assay of HSV-2: Prechilled Vero cells were infected with 200 PFU of HSV-2 for 2 h at 4oC. After removal of unbound virus cells were shifted to 37oC in presence and absence of serial dilution of extract. Test results shown dose dependent inhibition of plaque formation (77.78 and 55.56% inhibition in 50% ethanol and aqueou sextract respectively). Reduction in IC50 value was time dependent 12.61 and 33.74 µg ml-1 for 50% ethanol and aqueous extract respectively.

Post-attachment virus neutralization assay
In post attachment assay, a dose dependent inhibition of HSV-2 infection was recorded in both the extracts of S. pauciflorum (Figure 3). The ethanolic extract was more effective as compared to aqueous extract (71.5 and 31.5% inhibition at 50 μg ml-1 concentration respectively) at 72 h incubation period (Figure 3 A, 3B). However, 85.5% inhibition by 50% ethanolic extract from L. trinervis was observed after 72 h (Figure 3C), whereas aqueous extract did not show positive results (data not shown). Due to higher amounts of polyphenols and flavonoids in 50% ethanolic extracts may be one of the reasons of its higher activity on compared to aqueous extract (rich in saponins and triterpenoids; Pancholi, 2022).23
Figure 3
Effects of S. pauciflorum 50% ethanolic (A) and aqueous extracts (B) and L. trinervis 50% ethanolic extracts (C) on post- attachment assay of HSV-2: Prechilled Verocells were infected with 200 PFU of HSV-2 for 2 h at 4oC and after the removal of unbound virus cells were incubated with serial dilution of extract in different time period at 4oC. S. pauciflorum 50% ethanolic extract showed 71.5% inhibition at 50µg ml-1 concentration on 72 h incubation. On the other hand aqueous extract demonstrated 31.5% inhibition at 50 µg ml-1concentration with respect to Acyclovir (98.78% at 10µM concentration). L. trinervis 50% ethanolic extract showed 85.55% inhibition at 50 µg ml-1 concentration. However, aqueous extract does not show dose dependent inhibition upto 50 µg ml-1 concentration with respect to cell control (data not shown).

Conclusions
The present study has demonstrated that 50% ethanolic and aqueous extracts prepared from S. pauciflorum and leaves of L. trinervis possess potent in vitro activity against HSV-2. The TI values suggest that the antiviral activity of these plant extracts was not due to the cellular cytotoxicity. The 50% ethanolic extract from L. trinervis inhibit attachment of virus to host cells, prevent virus penetration and reduces HSV-2 infection even after virus attachment. These studies are encouraging and may help in devising alternate strategies for the treatment of HSV-2 infection. Further isolation of key compounds responsible for the Anti-HSV property is in active progress.
