Introduction
Cancer is a complex disease that arises from the uncontrolled proliferation of cells. It is one of the leading causes of death worldwide. The second most significant incidence of mortality in the United States and a serious global public health issue is cancer.1 In 2020, the COVID-19 pandemic had a negative impact on cancer detection and therapy. Delays in screening and therapy due to health care facility shutdowns and concern over COVID-19 exposure led in decreased access to care, which might cause a temporary decline in cancer incidence followed by a drastic increase in chronic cancer and, eventually, elevated mortality.2 Conventional cancer therapies such as chemotherapy, radiation therapy, and surgery have limited efficacy and are often associated with significant side effects. Probiotics can be a potential alternative therapy that is gaining popularity for treating cancer.
Probiotics are the living microbes which confer health benefits when consumed in enough quantities. They are well-known for their beneficial effects on the gastrointestinal tract, including the modulation of the gut microbiota and the enhancement of the immune system.3, 4 In recent years, several studies have suggested that probiotics may have antioncogenic potential, i.e., the ability to prevent or inhibit the development of cancer. Probiotics have been shown to exert their antioncogenic effects through various mechanisms, including the production of bacteriocins, modulation of the gut microbiota, induction of apoptosis, and inhibition of inflammation.5 Again, the incorporation of nanoparticles bestows different health modulatory effects.6, 7 However, there are several challenges associated with the use of probiotics as anticancer agents, including the lack of standardization of probiotic strains, the variability of probiotic effects among individuals, and the need for targeted delivery systems.
Despite these challenges, probiotics remain a promising alternative for cancer prevention and treatment. Future research should focus on the identification of specific probiotic strains and mechanisms of action that are effective against different types of cancer, the development of targeted delivery systems for probiotics, and the evaluation of the safety and efficacy of probiotics in clinical trials. The current study focuses on the antioncogenic probiotic strains and explored a better understanding on their anticancer mechanisms. The study will possibly provide ideas to the oncologists for designing probiotic based antioncogenic drugs by targeting the mode cancer inhibitory pathways of the potential probiotic strains.
Modern Life Style and Cancer
Cancer is a complicated condition that can be impacted by a number of variables, especially lifestyle issues. The chance of developing cancer is correlated with a number of lifestyle variables, according to several researches. For instance, there is a higher risk of cancer in those who smoke, drink too much alcohol, don't exercise, eat poorly, and are obese (Figure 1). Smoking is the leading cause of preventable cancer deaths worldwide, with a strong link to lung cancer, as well as several other types of cancer, including mouth, bladder, throat, kidney, and pancreatic cancer. According to the American Cancer Society, nearly one-third of all cancer deaths in the United States are caused by smoking.8 Excessive alcohol consumption is also linked to an increased risk of several types of cancer, including breast, liver, and colon cancer. Studies have shown that consuming more than two drinks per day for men and one drink per day for women increases the risk of cancer.9 Sedentary behavior and a deficiency of physical exercise have also been linked to a higher risk of developing cancer, including breast, colon, and lung cancer. Up to 25% less risk of colon and breast cancer can be achieved by physical exercise, as reported by the Global Cancer Research Fund.10 An unhealthy diet, particularly one that is high in processed and red meat, has been linked to an increased risk of several types of cancer, including colon, stomach, and pancreatic cancer. On the contrary side, a diet high in fruits, whole grains, and vegetables has been linked to a decreased cancer incidence.11 Obesity has been identified as a significant risk factor for several types of cancer, including breast, colon, and kidney cancer. According to the American Cancer Society, obesity may be responsible for up to 20% of all cancer deaths in the United States.12
Hence, a healthy lifestyle that includes regular physical activity, a balanced diet, limited alcohol consumption, and no smoking can help reduce the risk of cancer. These lifestyle factors, along with early detection and appropriate medical care, can contribute to better outcomes for cancer patients.
Factors Contributing Cancer in Indian Population
The most common types of cancer in India are breast, cervical, lung, oral, and colorectal cancer. Different studies suggest that the main causes of cancer in India are a combination of various factors, including tobacco use (both smoking and smokeless forms), unhealthy diet and lifestyle, exposure to environmental pollution, and infections such as human papillomavirus (HPV) and hepatitis B and C viruses (HBV and HCV).13, 14, 15 Therefore, prevention strategies that address these risk factors are crucial in reducing the burden of cancer in India.
Gut Microbiota and Cancer
The term "gut microbiota" describes the varied population of microorganisms, including bacteria, viruses, fungus, and protozoa that live inside human gut. Current researches indicate that the gut microbiota is critical to preserving human health and that dysbiosis, or an imbalance in the makeup or function of the gut microbiota, is linked to a number of disorders, including cancer. According to a number of studies, dysbiosis of the gut microbiota is linked to a higher risk of several cancers, including pancreatic, breast, and colorectal cancer.16 The vast ecology of microbes that live in the digestive tract makes up the human gut microbiota. Recent studies have demonstrated that the gut microbiota is crucial for several physiological functions, such as metabolism, immunological control, and digestion. Dysbiosis can cause immune system abnormalities, oxidative stress, and chronic inflammation, all of which can accelerate the growth and spread of cancer.17 Additionally, the makeup of the gut microbiota may affect the effectiveness of cancer therapies like chemotherapy and immunotherapy. Certain bacterial species have been reported to have both pro- and anti-tumor effects. Thus, altering the gut microbiota may be a promising technique for both preventing and treating cancer.18 To completely comprehend the intricate interplay between the gut microbiota, host variables, and environmental factors in cancer, further study is necessary to determine the precise mechanisms by which the gut microbiota promotes cancer formation and progression.
Probiotics: A Newer Approach to Fight Cancer
Probiotics are living microbes that could offer anti-cancer or anti-oncogenic benefits to the host. The capacity of probiotics to regulate the gut microbiota is one way they may have anti-oncogenic potential. In order to sustain immunological and intestinal homeostasis, the gut microbiota is essential. Many cancers, including colon, breast, and liver cancer, have been associated to dysbiosis or change of the gut microbiota. By encouraging the growth of helpful bacteria and stifling the growth of harmful bacteria, probiotics can aid in keeping up the equilibrium of the gut microbiota. 3 Probiotics have also been demonstrated to have immunomodulatory effects that improve the host's immunological response to cancer cells. For instance, some probiotic strains can increase the production of cytokines like tumor necrosis factor-alpha and interferon-gamma, which can trigger immune cells to target cancer cells. Moreover, probiotics can create metabolites like short-chain fatty acids (SCFAs), which have been demonstrated to have anti-cancer capabilities.19 In vitro and in vivo studies have revealed that SCFAs, which are formed by the gut microbiota through the fermentation of dietary fibres, suppress the development and proliferation of cancer cells. Last but not least, probiotics have been proven to directly combat cancer. For instance, certain probiotic strains can create bacteriocins, which are tiny peptides that can stop the development of cancer cells.20, 21 The capacity of probiotics to modify the intestinal flora, boost immune response, create anti-cancer metabolites also bestow anti-cancer benefits. To completely comprehend the mechanisms of action and the effectiveness of probiotics as an anti-cancer agent, additional study is nonetheless required.
Mode of Antioncogenic Actions
Several studies have suggested that probiotics have potential anti-cancer properties, particularly in preventing and treating certain types of cancers. The probiotic specific modes of antioncogenic responses are shown in Table 1. Here are some ways in which probiotics may have antioncogenic potentials:
Modulating the gut microbiome
Probiotics can modulate the gut microbiome, which has been linked to the development of certain cancers. By promoting the growth of beneficial bacteria, probiotics may help prevent the development and spread of harmful bacteria that can damage the gut lining and lead to inflammation and cancer.22
Reducing inflammation
Chronic inflammation is a chief attribute for the development of cancer. Probiotics have been shown to reduce inflammation in the gut and other parts of the body, which may help prevent the development of cancer.4, 23
Enhancing the immune system
Probiotics can stimulate the immune system, which plays a critical role in identifying and eliminating cancer cells. By enhancing the immune response, probiotics may help prevent the progress of cancer cells.24, 25
Producing anti-cancer compounds
Some strains of probiotics produce compounds that have been shown to have anti-cancer properties. For example, some strains of Lactobacillus produce a compound called Lactobacillin, which has been shown to inhibit the growth of colon cancer cells.24
Table 1
Probiotic specific antioncogenic responses
Probiotics against Different types of Cancers
Some studies suggest that certain strains of probiotics may have a beneficial effect on certain types of cancer when used as an adjunct to conventional cancer treatment. For example, some research suggests that Lactobacillus acidophilus and Bifidobacterium lactis may help reduce the risk of colorectal cancer and improve the effectiveness of chemotherapy in patients with colon cancer.4, 36, 37, 38 Another study showed that a specific strain of probiotic called Lactobacillus reuteri may help reduce the risk of breast cancer in mice by altering the gut microbiome and enhancing the immune system's ability to fight cancer cells.26 It's worth noting that more research is needed to determine the effectiveness of probiotics for preventing or treating cancer, and that the use of probiotics as a cancer treatment should always be discussed with a healthcare provider. Some types of cancers that have been studied for the potential use of probiotics in treatment or prevention include:
Colorectal cancer
Several studies have recommended that certain strains of probiotics, such as Lactobacillus acidophilus, Bifidobacterium bifidum, and Lactobacillus rhamnosus GG, can inhibit the development of colorectal cancer and reduce its recurrence.36, 37, 38
Breast cancer
Animal studies have shown that certain probiotics, such as Lactobacillus casei, can inhibit the growth of breast cancer cells.39, 40
Gastric cancer
Clinical studies have suggested that the administration of probiotics may reduce the risk of gastric cancer development. Bifidobacterium, Lactobacillus, and Streptococcus species were found to be effective.41
Liver cancer
Some preclinical studies have shown that certain strains of probiotics, such as Lactobacillus rhamnosus and Bifidobacterium lactis, can inhibit the growth of liver cancer cells.4
Cervical cancer
Modern epidemiological as well as experimental studies have shown a striking association between higher probiotic intake and slowed cancer development, and Lactobacilli have been demonstrated to have the capacity to suppress the growth of numerous types of cervical cancer cells.15
It's crucial to note that probiotics cannot replace conventional cancer treatments such as chemotherapy, surgery, and radiation. However, they may have potential as adjunctive therapy to improve the efficacy of cancer treatments and reduce side effects.
Mode of Action of Antioncogenic Components Produced by Probiotics
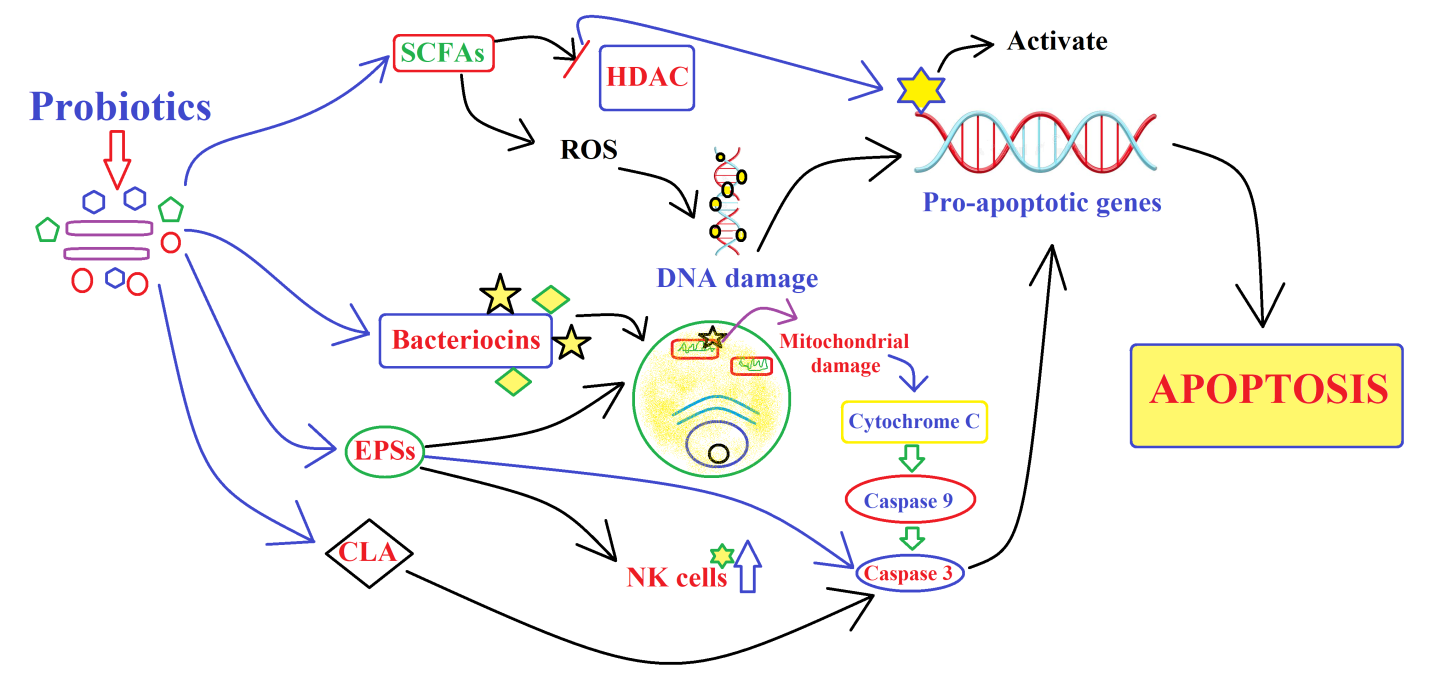
Probiotics have been shown to secrete various substances that have anti-cancer properties (Figure 2). Probiotics secrete various Short-chain fatty acids, Bacteriocins, Exopolysaccharides, and Conjugated linoleic acid which have potential antioncogenic activities.
Short-chain fatty acids (SCFAs)
SCFAs such as butyrate, propionate, and acetate, which have been shown to inhibit the growth of cancer cells and promote apoptosis of cancer cells.42 SCFAs have been shown to induce apoptosis in cancer cells through various mechanisms. One potential mechanism is the inhibition of histone deacetylase (HDAC) activity. SCFAs can inhibit HDAC activity, which can lead to increased acetylation of histones and subsequent activation of pro-apoptotic genes.19 SCFAs can also induce the release of reactive oxygen species (ROS) in cancer cells, which can cause DNA damage and trigger apoptosis.43 By encouraging the synthesis of pro-inflammatory cytokines that can cause cancer cells to undergo apoptosis, SCFAs can also modify the immune system. Finally, SCFAs can impact how genes involved in cell survival and proliferation are expressed, which lowers cell viability and promotes apoptosis of cancer cells.44, 45
Bacteriocins
Bacteriocins, the antimicrobial peptides, synthesized by some probiotic bacteria, have shown to have anti-cancer activity against various types of cancer cells.20, 21 There are various potential ways for how bacteriocins destroy cancer cells, while the precise processes are still not entirely known. Bacteriocins have been shown to induce apoptosis in cancer cells by activating caspases, which are enzymes that play a key role in the initiation of apoptosis. Bacteriocins can disrupt the cancer cell membrane, leading to cell lysis and death. Bacteriocins can inhibit DNA synthesis in cancer cells, which can lead to cell death. Bacteriocins can stimulate the immune system to recognize and eliminate cancer cells.46 The activation of caspases is a key step in the induction of apoptosis by bacteriocins. The exact mechanism by which bacteriocins activate caspases may vary depending on the specific bacteriocin and the type of cancer cells being targeted. Yet, research indicates that bacteriocins can activate caspases by tampering the mitochondrial membrane potential, which releases cytochrome C and activates caspase-9. Activated caspase-9 then cleaves and activates downstream effector caspases, such as caspase-3, leading to the initiation of apoptosis. Additionally, some bacteriocins may activate caspases through other mechanisms, such as the activation of death receptors or the inhibition of anti-apoptotic proteins.47, 48 The exact mechanism may be different for different bacteriocins and requires further study.
Bacteriocins do not have the ability to sense cancer cells specifically. However, some bacteriocins have been shown to have selectivity towards cancer cells as contrasted to normal cells, which could be due to divergences in their cell membranes, metabolism, or other factors. For example, some bacteriocins have been found to selectively target cancer cells with altered lipid metabolism or higher expression of certain receptors compared to normal cells. Additionally, some bacteriocins have been modified or engineered to target cancer cells specifically by incorporating cancer cell-specific ligands or peptides into their structure.46
Exopolysaccharides (EPSs)
EPSs are complex carbohydrates produced by some probiotic bacteria that have been shown to have anti-inflammatory and anti-cancer properties.49, 50 Exopolysaccharides (EPS) produced by probiotics have been reported to exert anti-cancer properties through various mechanisms such as inducing apoptosis, inhibiting proliferation, and suppressing angiogenesis in cancer cells. EPS can also modulate the immune system to enhance the host's anti-tumor response. For example, a study showed that EPS produced by Lactobacillus plantarum KF5 isolated from kimchi inhibited the growth of human colon cancer cells by inducing apoptosis and inhibiting cell cycle progression. The authors suggested that the anti-cancer activity of EPS may be mediated through the activation of caspase-3 and downregulation of Bcl-2, which are known to be involved in the regulation of apoptosis.51 Another study reported that EPS produced by Bifidobacterium animalis subsp. lactis CP9 enhanced the anti-tumor effect of natural killer (NK) cells against human colon cancer cells in vitro. The authors suggested that the anti-cancer activity of EPS may be due to its ability to activate NK cells through the upregulation of interleukin-12 (IL-12) and interferon-gamma (IFN-γ).52 It has been suggested that EPS may activate caspases through the intrinsic or extrinsic apoptotic pathways, or by directly targeting the mitochondrial membrane potential, which can lead to the release of cytochrome C and activation of caspase-3. However, further investigation is desirable to elucidate the mechanism behind this process entirely.53, 54, 55
Conjugated linoleic acid (CLA)
CLA is a fatty acid produced by some probiotic bacteria that had been evaluated to inhibit the growth of cancer cells and induce apoptosis.56, 57 CLA has been found to induce apoptosis in various cancer cell lines by caspase activation, which are key enzymes involved in the initiation of apoptosis. One study investigated the apoptotic effects of CLA in human colon cancer cells and found that treatment with CLA resulted in the activation of caspase-3 and caspase-9, leading to apoptosis.58 Another study on CLA-induced apoptosis in breast cancer cells found that the expression of caspase-3 and caspase-8 was upregulated and caspase-9 was activated, leading to apoptosis.59
Probiotics as Apoptosis Inducer
Probiotics are live microorganisms that have been shown to induce apoptosis, or programmed cell death, in cancer cells. The exact mechanisms by which probiotics induce apoptosis are still being studied, but several possible mechanisms have been proposed. One mechanism by which probiotics induce apoptosis is through the production of intracellular reactive oxygen species (ROS). ROS are highly reactive molecules that can cause damage to cellular components, including DNA and proteins, and can trigger apoptosis. Previous studies found that probiotics, such as Lactobacillus rhamnosus and Lactobacillus plantarum, can induce apoptosis in cancer cells by increasing intracellular ROS production.60, 61 Another mechanism by which probiotics induce apoptosis is through the activation of caspases, which are enzymes that play a key role in the apoptotic process. Probiotics have been shown to activate caspases in cancer cells, leading to apoptosis.61 Furthermore, probiotics may also modulate cell cycle progression, leading to apoptosis in cancer cells. Previous studies found that Lactobacillus rhamnosus supernatant inhibited the growth of human colon cancer cells by inducing G1 cell cycle arrest and apoptosis.60, 61 Overall, probiotics have been shown to induce apoptosis in cancer cells through a variety of mechanisms, including the production of intracellular ROS, activation of caspases, and modulation of cell cycle progression. These findings suggest that probiotics may have potential as a therapeutic approach for cancer, although further research is needed to fully understand the mechanisms involved and their clinical implications.
Probiotics have been shown to activate caspases, which are enzymes that play a key role in the apoptotic process. One proposed mechanism by which probiotics activate caspases is by stimulating the regulation of pro-apoptotic proteins (Bax), and downregulating anti-apoptotic proteins (Bcl-2), in cancer cells.62 Probiotics, such as Lactobacillus acidophilus and Lactobacillus casei, have been shown to induce apoptosis in colon cancer cells by upregulating the expression of Bax and downregulating the expression of Bcl-2. This results in an increase in the ratio of Bax/Bcl-2, which triggers the activation of caspase-3 and subsequent apoptosis.63 In addition, probiotics have also been shown to activate caspases through the extrinsic apoptotic pathway. Probiotic bacteria, such as Lactobacillus rhamnosus, have been found to induce apoptosis in cancer cells by upregulating the expression of death receptors, such as Fas and DR5, on the surface of cancer cells. This leads to the activation of caspase-8, which initiates the extrinsic apoptotic pathway and subsequent activation of caspase-3.64 Hence, probiotics have been shown to activate caspases in cancer cells through numerous pathways, including upregulation of pro-apoptotic proteins and death receptors, downregulation of anti-apoptotic proteins, and subsequent activation of caspase-3. However, further research is needed to fully elucidate the mechanisms by which probiotics activate caspases and their potential as a therapeutic approach for cancer.
Antioncogenic Activities of the Genus Bacillus
The genus Bacillus gains enormous significance in microbiology due to their ability to produce spores and endurance in non-favorable environments. There is some evidence to suggest that certain strains of Bacillus probiotics may have anticancer properties, although further research is needed to confirm their efficacy and safety for cancer prevention and treatment. It had been found that a probiotic mixture containing Bacillus subtilis and Enterococcus faecium was effective in reducing the incidence and severity of chemotherapy-induced diarrhea in patients with colorectal cancer. The researchers suggested that the probiotic may have exerted its protective effect by modulating the gut microbiota and enhancing immune function.65
Further, it had been found that a Bacillus probiotic (Bacillus amyloliquefaciens X030) was effective in inhibiting the growth of colon cancer cells in vitro. The researchers suggested that the probiotic may have exerted its anticancer effect by producing a compound called Bacillomycin Lb, which has been shown to have antitumor activity.66
However, it is important to note that these studies are preliminary and more research is needed to determine the potential use of Bacillus probiotics in cancer prevention and treatment. It is always recommended to consult with a healthcare professional before using any dietary supplement, including probiotics, as a form of cancer treatment or prevention.
Nanocoating of Probiotics for Targeting Cancer Cells
The incorporation of nanoparticles with probiotics may pave an innovative path for targeting different types of cancers (Table 2). The potential of Lactobacillus plantarum A7, as an antioncogenic agent to reduce the viability of colon cancer cells may be enhanced by coating the strain with chlorin e6-nanoparticles67 to target the delivery of the probiotics to the cancer cells, and will possibly show that the combination of probiotics and nanoparticles significantly reduce the viability of colon cancer cells. Again, chitosan nanoparticles are extensively employed as drug delivery agents68 and hence, may be used to improve the therapeutic efficacy of Lactobacillus acidophilus against colon cancer. The chitosan nanoparticles will improve the viability and stability of the probiotic strain and will possibly enhance its anticancer effects against colon cancer cells. The combination of Lactobacillus acidophilus and chitosan nanoparticles will certainly induce apoptosis in colon cancer cells and inhibit their growth more effectively than the probiotic alone. Nanoparticles have been shown to enhance drug delivery to cancer cells and increase the efficacy of anticancer agents. The chitosan nanoparticles may also facilitated the delivery of the probiotic to colon cancer cells, leading to a more targeted and effective treatment. However, invitro and invivo researches are needed to establish the hypothesis and to elucidate the exact mechanisms of action involved.
Table 2
Nanocoating of probiotics and their efficiencies against different cancers
Antioncogenic Fungi
Fungi are a diverse group of microorganisms that have been studied for their potential use as probiotics in cancer prevention and treatment. Recent research has shown that certain fungal strains can modulate the gut microbiota, reduce oxidative stress, and stimulate the immune system, which can have anti-tumor effects. One of the most studied fungal strains for its potential use as a probiotic is Saccharomyces cerevisiae. Studies have shown that S. cerevisiae can also inhibit human colon cancer cells.74 Similarly, Wang et al.75 investigated the fungal communities in the gastrointestinal tract of tumor patients and found that certain fungal strains were more prevalent in patients with colorectal cancer. Other fungal strains, such as Fusarium venenatum76 and Pleurotus ostreatus,77 have also been investigated for their potential use as probiotics in cancer aversion and therapies. Fusarium venenatum can reduce oxidative stress, suggesting a potential anti-tumor effect. Similarly, a study by Jedinak et al.78 investigated the effect of Pleurotus ostreatus on the growth of human colon and breast cancer cells and found that it had anti-tumor effects through p53-dependent and p53-independent pathway. Further study is required to completely comprehend the potential advantages and mechanisms of action of fungal probiotics, even if these trials indicate possibilities for their application in cancer prevention and therapy. It is also important to note that the safety and efficacy of fungal probiotics need to be further evaluated before they can be used in clinical settings. Overall, fungal probiotics represent a promising area of research in the fight against cancer, and more studies are needed to explore their potential use as anticancer agents.
Antioncogenic Algae
Algae are a diverse group of aquatic organisms that have been studied for their potential use as probiotics in cancer prevention and treatment. Recent research has shown that certain algal strains can modulate the gut microbiota, reduce inflammation, and stimulate the immune system, which can have anti-tumor effects. One of the most studied algal strains for its potential use as a probiotic is Chlorella vulgaris. Studies have shown that Chlorella vulgaris can modulate the gut microbiota and reduce inflammation, which may have a protective effect against colorectal cancer.79 Similarly, Zhang et al.80 investigated the effects of the exopolysaccharides of Chlorella pyrenoidosa FACHB-9, Scenedesmus sp. and Chlorococcum sp. on human colon cancer cells and found that they had anti-tumor effects in vitro. Other algal strains, such as Spirulina platensis and Dunaliella salina, have also been investigated for their potential use in cancer prevention and treatment. Fayyad et al.81 showed that the methanolic extracts of Spirulina platensis have anti-tumor effects against human cancer cell lines (L20B and MCF7). Similarly, Singh et al.82 investigated the anti-tumor effects of gold nanoparticles from Dunaliella salina and found that it had potent cytotoxic activity against human breast cancer cell lines. Additional investigation is necessary to completely comprehend the potential advantages and mechanisms of action of algal probiotics, yet these results indicate possibilities for their application in cancer prevention and therapy. It is also important to note that the safety and efficacy of algal probiotics need to be further evaluated before they can be used in clinical settings. Overall, algal probiotics represent a promising area of research in the fight against cancer, and more studies are needed to explore their potential use as anticancer agents.
Key Challenges
While the potential of probiotics as anticancer agents is promising, there are several challenges and limitations that need to be addressed in order to develop effective and safe probiotics for cancer prevention and treatment. Some of the key challenges include:
Standardization of probiotic strains
There is a lack of standardization in the selection and characterization of probiotic strains, which makes it difficult to compare results across studies and establish the optimal dose and duration of probiotic therapy.
Survival and stability of probiotics
Probiotics must be able to survive the acidic environment of the stomach and the bile salts of the small intestine in order to reach the colon, where they exert their effects. In addition, probiotics must remain stable and viable during storage and transportation, which can be challenging.
Safety concerns
Although probiotics are generally considered safe, there are concerns about their safety in certain populations, such as immunocompromised individuals and those with underlying medical conditions. In addition, the potential for probiotics to transfer antibiotic resistance genes to pathogenic bacteria is a concern that needs to be addressed.
Limited understanding of mechanisms of action
While there is some evidence to suggest that probiotics can modulate the gut microbiota, reduce inflammation, and stimulate the immune system, the exact mechanisms of action are not fully understood.
Lack of clinical evidence
While there are some promising preclinical studies, there is a lack of robust clinical evidence to support the use of probiotics in cancer prevention and treatment.
To address these challenges, future research should focus on standardization of probiotic strains, improving probiotic survival and stability, addressing safety concerns, increasing understanding of mechanisms of action, and conducting well-designed clinical trials to establish the efficacy of probiotics in cancer prevention and treatment. Additionally, alternative approaches such as the use of postbiotics, phages, and engineered probiotics may offer new avenues for developing effective and safe anticancer therapies.
Conclusion
The antioncogenic potential of probiotics is a promising area of research that has gained significant interest in recent years. A regular probiotic based diet may stimulate the antioncogenic responses in the body. However, several challenges exist, including the identification of specific strains with potent antioncogenic effects, the determination of optimal dosage and duration of treatment, and potential risks associated with the use of live microorganisms. Furthermore, the heterogeneity of cancer types and patients' microbiomes further complicate the selection of probiotics for clinical applications. The development of alternative approaches, such as the use of microbial-derived components, genetically modified probiotics, and synthetic biology, may provide potential solutions to overcome these challenges and enhance the antioncogenic potential of probiotics. Future research in this area should continue to explore these alternative approaches to advance the potential of probiotics in cancer prevention and treatment. Overall, the antioncogenic potential of probiotics holds great promise, and with further research and development, they may become a valuable tool in the fight against cancer.