Introduction
Plants are the earliest source of pharmacologically active chemicals and have supplied mankind with several medicinally beneficial molecules for generations. Today, it is believed that more than two-thirds of the global population uses medications derived from plants. Plants have been the most important source of natural compounds for disease treatment research. As well as contributing to aging, free radicals are at the root of many disorders associated with older years.1 Free radical cell damage has been linked to many different diseases, including cancer, arthritis, atherosclerosis, Alzheimer's, and diabetes.2 Some evidence shows that cell death mechanisms within the body, such as apoptosis and in severe cases necrosis, are initiated and enhanced by reactive nitrogen species and free radicals.3 Targeted antioxidants, like mitochondria-targeted ubiquinone, may reduce the damage to the liver caused by drinking too much alcohol and lead to better therapeutic effects.4 The plant Acmella oleracea (L.) R. K. Jansen, sometimes referred to as Spilanthes acmella var. oleracea, is a plant that belongs to the family Asteraceae and may be found in the northern part of Brazil. The population uses their leaves and flowers in folk medicine and as a flavoring in traditional cuisines.5 It is a flowering herb which is native to South America and is known there as jambù. In modern times, it is utilized all over the world for a variety of applications, including pest management, food, cosmetics, and pharmaceuticals.6 In traditional medicine, jamb preparations are used to heal wounds and treat rheumatism. They are also utilised as analgesics, anticonvulsants, antipyretics, antimalarials, and febrifuges.7 Most of the time, toothaches and sore throats are treated with anesthetics made from this plant's leaves and stems.8 This plant has a variety of secondary metabolites, with the N-alkylamides being primarily responsible for its biological features. Spilanthol, a member of this class of chemicals, has demonstrated, among other things, a high pesticidal efficiency9 due to its ability to enter insects' central nervous systems (CNS).10 Acmella oleracea is the only member of the genus Acmella and may be responsible for much of its biological activity due to the presence of lipophilic N-alkylamines.11 The primary alkamide in the aerial sections, spilanthol (affinin), is what gives the plant its medicinal properties, such as anaesthetic12 and anti-inflammatory effects,5 immunological stimulation,13 and saliva-secretion induction. We have chosen the methanol extract of Acmella oleracea leaves to examine the antioxidant, cytotoxic, thrombolytic, and antibacterial contents. A. oleracea is regarded as a good substitute for a number of commercial medications due to its wide range of traditional uses and pharmacological potential.
Therefore, we have chosen the methanol extract of Acmella oleracea leaves to evaluate its antioxidant, cytotoxic, thrombolytic, and antibacterial properties. A. oleracea is recognised as a possible alternative to numerous commercially available drugs due to its broad traditional usage and pharmacological potential. In the following phrase, we will discuss the results of our investigation into the antioxidant, cytotoxic, thrombolytic, and antibacterial characteristics of A. oleracea.
Materials and Methods
Plant materials
The plant Acmella oleracea leaves was obtained from Dhanmondi Lake, Dhanmondi, Dhaka, Bangladesh (23° 44' 47.2776'' N, 90° 22' 33.6540'' E). The plant sample was approved by the department of pharmacy, Bangladesh University. Date and location of collecting of each specimen were recorded alongside the specimen numbers. The gathered leaves were dried for about seven days at room temperature and out of direct sunshine. A flow chart outlines the whole approach for the research shown in Figure 1.
Preparation of the crude extract
Cold extraction (Methanol extraction)
This plant thrives in dark, rich soil with plenty of organic matter. If planting seeds outside, they should not be exposed to cold, thus do it after the last frost. To germinate, seeds require direct sunshine, thus they should not be buried.14
The desired components, plants, or plant sections were removed from the gathered plant parts (leafs). They were cut into little pieces and sun dried for a week. A suitable grinder was used to grind the plant parts into a coarse powder. Until the analysis started, the powder was kept in a container that was kept cool, dark, and dry. 180 gram of powdered material were soaked in 1000 milliliter of 90% methanol in a clean glass container with a flat bottom. During ten days, the container and its contents were sealed and preserved with periodic shaking and stirring. Following that, the whole combination was rough filtered through a piece of white, cotton cloth that had been cleansed. After that, Whatman filter paper was used to filter the water. In order to get crude extract, the filter was left outside to let the solvent evaporate.
Antioxidant activity evaluation
In a quantitative experiment, the antioxidant activity of the extract was determined based on its capacity to scavenge the stable DPPH free radical.
DPPH test for calculating total antioxidant capacity
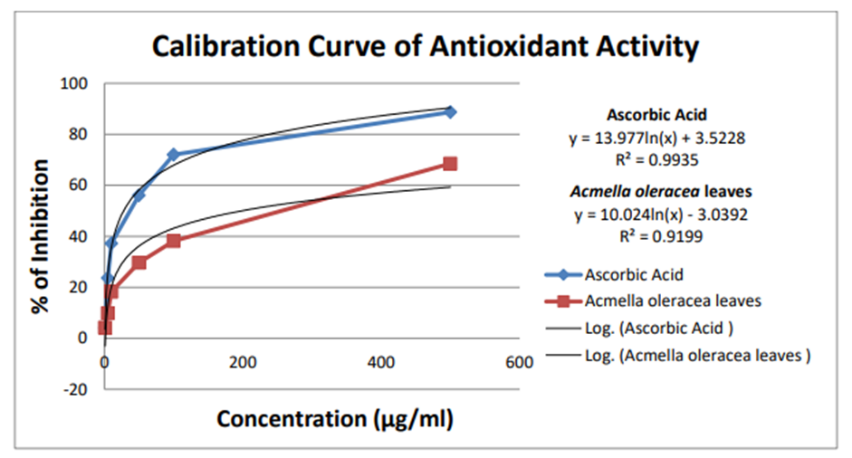
According to the findings of Sadhu et al., the scavenging of the stable 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical may be used as a measure of a substance's anti-oxidant activity. A. oleracea extracts' antioxidant activity was determined using the redox potential of DPPH. The DPPH molecule is a persistent free radical that exhibits a delocalized spare electron and a bright violet color in the methanol absorption band at 520 nm. Its dark vio-let color turns into yellow when reduced (trapped by the tested products). The DPPH reduction operation of each extract was determined using the procedure outlined by Zuraini et al. Methanol extract of A. oleracea leaves was tested for its ability to scavenge free radicals using DPPH free radical as a biomarker (Braca et al., 2001a). A total of 3 millilitres of a methanolic DPPH (0.004%) solution was added to 0.1 millilitres of the crude extract (M. S. Ali et al., 2013). The absorbance at 517 nm was measured after 30 minutes (Figure 2), and the percentage of inhibition effect was determined using the following equation.
% of inhibition = Blank absorbance – Solution absorbance/Blank absorbance × 100
The absorbance decreases as the free radical scavenging activity increases.
Reducing power
Oyaizu's (1986) method was used to determine the reducing power using ascorbic acid as the reference compound. One milliliter to five hundred milliliters of concentrated extracts were combined with two and a half milliliters of phosphate buffer (pH 6.6), two and a half milliliters of potassium ferricyanide, and one milliliter of distilled water. At a temperature of 50°C then the mixture was incubated. After adding 2.5 milliliters of 10% trichloroacetic acid to the mixture, it was then centrifuged at 3000 revolutions per minute for ten minutes. UV spectroscopy at 700 nm was used to measure the absorbance of the top layer of the solution after adding 2.5 ml of distilled water and 0.5 ml of newly prepared (0.1%) ferric chloride solution. A stronger reducing power might be inferred from the reaction mixture's increased absorbance. The reference solution was ascorbic acid, while the blank solution was 1ml methanol phosphate buffer (pH 6.6).
Thrombolytic activity test
We used Prasad et al (2007) described procedure to test the thrombolytic activity of the leaf extract in vitro. Blood was obtained from healthy individuals who had never used oral contraceptives or anticoagulants, and 1.0 ml of venous blood was placed to weighted micro centrifuge tubes to clot. The thrombolytic activity of all extractives was assessed using a technique devised using streptokinase (SK) as the control drug. Overnight, each 100 mg of plant extract was mixed with 10 mg of distilled water. After that, a 0.22-micron syringe filter was used to separate the soluble supernatant from the rest of the mixture. For the clot lysis technique, healthy individuals' vein blood was taken and split into numerous pre-weighed sterile micro centrifuge tubes (1 ml/tube). The tubes were then incubated for 45 minutes at 37 degrees Celsius. After clot formation, the serum was completely removed without disturbing the clot, and the clot weight was estimated by remeasuring the weight of each tube containing the clot (clot weight = weight of clot-containing tube minus weight of tube alone). Each micro centrifuge tube had a pre-weighed clot in it, and 100l of each type of partitioning agent's aqueous solution and crude extract were added individually. Thereafter, 100 ml of streptokinase and 100 ml of distilled water were added to the positive and negative control tubes, respectively. After incubating all tubes at 37° C for 90 minutes, any clot lysis was seen. Within a week of incubation, the discharged fluid was collected and the tubes were weighed once again to assess the difference in weight caused by clot breakup. As demonstrated below, the percentage of clot lysis was based on the difference between the weights before and after clot lysis:
% of clot lysis = 1st clot weight – 2nd clot weight / 1st clot weight × 100
Cytotoxicity screening
Brine shrimps
The brine shrimp lethality bioassay is an effective and simple cytotoxicity test for bioactive substances. Its effectiveness is determined by testing substances on brine shrimp (Acmella oleracea), a straightforward zoological creature.15 The brine shrimp lethality bioassay is commonly used to determine the toxicity of heavy metals, pesticides, pharmaceuticals, especially natural plant extracts, and other chemicals.16
Brine shrimp lethality bioassay
The plant's cytotoxic activity was assessed using the Brine shrimp lethality bioassay technique, with six graded doses (200 g/mL, 100 g/mL, 50 g/mL, 20 g/mL, 10 g/mL, and 5 g/mL) utilized. The embryonic stages of the brine shrimp, Artemia salina Leach (Ocean 90, USA), were used as test organisms. Before hatching, eggs were incubated for 48 hours in brine containing a constant oxygen supply. After that, the adult nauplii were employed in the experiment. Both as a solvent and in the role of a negative control, DMSO was used throughout this experiment. In this situation, vincristine sulfate was employed as a reference standard. After 24 hours, the surviving was counted. If larvae did not move inside or externally during many seconds of observation, they were presumed dead. Food was not given to the larvae. We compared the dead larvae in each treatment to the dead larvae in the control to confirm that the mortality found in the bioassay may be attributable to bioactive substances rather than hunger.
Antimicrobial activity
The experiment followed the guidelines and practices set out by the Clinical and Laboratory Standards Institute (CLSI), using two strains of bacteria, one of which was Gram-positive (Staphylococcus aureus, ATCC 25923) and the other Gram-negative (Escherichia coli, ATCC 25922).17 Disk diffusion was used to measure the plant extracts' antibacterial activity against the test species, while Kanamycin discs (30 g/disc) served as a baseline for comparison.18 Investigations into the antibacterial properties of natural compounds and plant extracts frequently employ disc diffusion techniques. These tests rely on the use of discs as reservoirs holding solutions of the chemicals under investigation. However, a high concentration or volume is required for solutions with a low activity. After putting the sample-containing paper discs onto the plates, they were incubated at (352) °C for two days to determine the zones of inhibition. The existence of antibacterial activity was shown by the formation of clear inhibition zones around the discs.
Collection of microorganisms
Bacillus subtilis, Staphylococcus aureus, Salmonella typhi, and Vibrio mimicus were the bacterial species employed in this investigation. These were obtained as pure cultures from the pharmacy department of Bangladesh University in Dhaka.
Table 1
Antibacterial activity of all gram positive and gram negative bacteria of methanol extract of Acmella oleracea leaves
Results
DPPH radical scavenging assay
DPPH is typically employed as a reagent for the purpose of determining the antioxidants' ability to absorb free radicals.19 It's a free radical that can be stabilised by adding an electron or a hydrogen radical as a donor.20 It was shown that the extract of A. oleracea had a concentration-dependent increase in its ability to quench DPPH radicals (Figure 2) and the calibration curves showed that strong positive logarithmic correlation (r) which was close to +1. The methanol extract of Acmella oleracea leaves has moderate anti-oxidant activity. The IC50 of the Acmella oleracea leaves was 198.34µg/ml, whereas IC50 of Ascorbic Acid was 7.8µg/ml (Table 2). The experimental data for this species indicates that the extract likely possesses free radical scavenging properties. The outcomes and techniques of investigation of antioxidant activity were consistent with those of other research.
Table 2
Data for standard (Ascorbic Acid) and extract of Acmella oleracea leaves
Thrombolytic activity
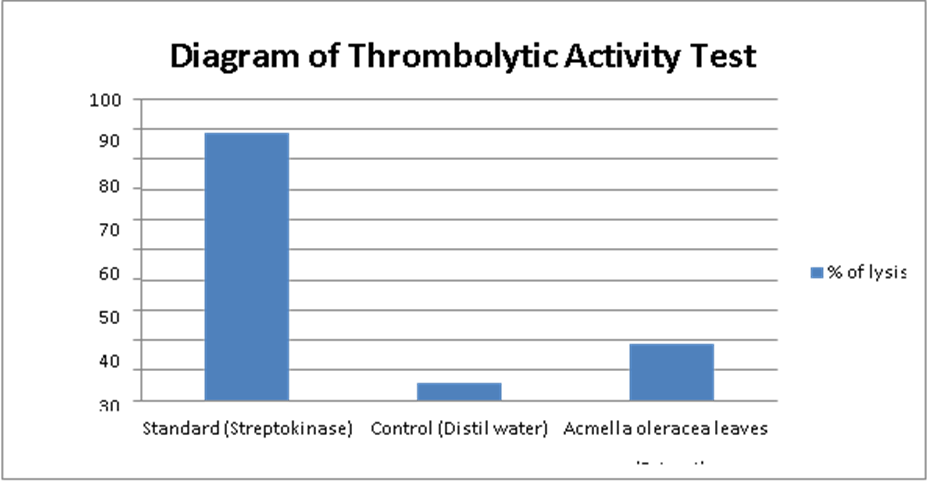
A. oleracea extract demonstrated modest clot lysis efficacy in a thrombolytic assay (Table 3) 18.69% (Figure 3) in terms of 88.49% lysis of clot for Streptokinasea positive control (Figure 4) in addition to 5.70% lysis were obtained for negative control (sterile distilled water). Apparently the methanol extract of A. oleracea leaves increased clot lysis (P<0.001) in comparison to the control group.
Table 3
Thrombolytic activity (in terms of % clotlysis) of Acmella oleracea leaves
Cytotoxic activity
Brine shrimp of LC50 values obtained from a lethality test revealed that A. oleracea extract and the extracts decreased toxicity towards the brine shrimp study. The LC50 values for the plant extracts ranged between 8.447 and 60.32 g/mL, treated as the significance of the toxicity of the extract of a plant. We found LC50 of A. oleracea was 1.431 µg/ML, LC90=91.65 µg/ML. So, this extract cannot be a progressive solution for further study as a potential anticancer agent generated from plants.
Antimicrobial activity
The present study indicated that methanolic extracts of Acmella oleracea were tested for their antibacterial activity using the disc diffusion technique utilising dried extracts. Additionally, the extract was put to the test against various strains of bacteria, comprising both gramme positive and gramme negative bacteria, and with the zone of inhibition being assessed in millimetres as well. Different antibacterial activity of different extracts against tested microorganism were given in the Table 1. Vibrio Mimicus a gram negetive bacteria showed excellent activity as it showed more inhibition zone in the Methanol and ethyl acetate extract rather than the other microorganism. In case of Escherichia coli bacteria, it showed comparative activity with standard drug. Salmonella typhi and Staphylococcus Aureus gave a moderate activity where Bacillus subtilis, was given slightly less activity with the extracts from other microorganisms.
Discussion
Plants provide a wealth of potentially useful structures for the development of new chemotherapeutic medicines. Therefore, Acmella oleracea's antibacterial chemicals may be effective against multidrug-resistant bacteria since they kill germs by a different method than antibiotics.21 According to the results, the antibacterial effect of the extracts is, most of the time, gram-negative bacteria are just as strong or stronger than gram-positive bacteria. These results are inconsistent with those of earlier tests of medicinal plants for antibacterial effects, wheremost of the active extracts were exclusively effective against Gram-positive bacteria. Inhibition zones may vary in size for many different plant species, depending on parameters such the extract's solubility in the medium, the substance's antimicrobial activity, and the growth and metabolic activity of the bacteria present.22 On the other hand, the fact that the antimicrobial action was shown to be effective against both Gram-positive and Gram-negative bacteria is strongly suggestive of the presence of antibiotic compounds that have a broad spectrum of activity. For instance, the extract of Acmella oleracea gave high antibacterial activity against Vibrio Mimicus. Where, the extracts showled moderate activity against Salmonella Typhi and Escherichia coli bacteria. Furthermore, Bacillus Subtilies showed little or no activity to the extract. The brine shrimp lethality bioassay, more often referred to as the BSLB, has been extensively utilised in the preliminary screening of crude extracts and isolated compounds for the purpose of determining the levels of toxicity that they exhibit towards brine shrimp. This study might potentially offer an indicator of the test materials' potential cytotoxicity. It has been widely employed in the initial screening of extracts and isolated compounds to determine the toxicity against brine shrimp, which might also serve as an indicator of the probable cytotoxic effects of the test materials. According to the result in this study the brine shrimp bioassay showed that the extracts of Acmella oleracea fraction process a little bit cytotoxic activity which cannot be regrade as lethal.
Previous studies had demonstrated that the antioxidant capacity of A. oleracea was dependent on the concentration of the extract, with the level of concentration being directly related to the level of activity. The DPPH radical scavenging assay, total antioxidant content, total phenolic and flavonoid contents, nitric oxide scavenging assay, and reducing power studies were used to assess this. The DPPH free radical scavenging activity technique was utilised in this investigation in order to examine the antioxidant activity of the methanol extract of Acmella oleracea leaves. In vitro free radical scavenging activity test was carried out with an extract of A. oleracea (leaves) that was dissolved in methanol. The results of this test demonstrated the presence of possible antioxidant activity. Typically, reducing power essay is employed to measure an antioxidant's capacity to give electrons.
The ability of the plant extract parts to reduce may be a key indicator of their possible antioxidant action.23 Some studies find that it has a close connection with antioxidant activity. It has been suggested that a compound's reducing capacity value is a potential indicator of its potent antioxidant action. Several investigations have demonstrated that the chemical's reducing properties are a result of the presence of reductants, and that its antioxidant properties result from the donation of hydrogen to break the chain of free radicals. In general, phenolic chemicals are regarded as antioxidants that break solid chains.24 But nonetheless, several authors have demonstrated a link between antioxidant activity and phenolic content, demonstrating that the antioxidative impact is directly related to phenolic compounds.25 Platelets play a crucial function in atherothrombosis by attaching to the damaged areas (produced by reactive oxygen species) of the endothelium surface.26 The activated platelets create platelet-to-platelet linkages and also attach to leucocytes, incorporating them into the complicated process of plaque formation and expansion.27 In comparison to the lysis percentage of its extract, the thrombolytic activity of A. oleracea was very high. Due to its promoting action, it may be used as an anti-thrombotic medication in the treatment of cardiovascular diseases.
Conclusion
From the result section, it is shown that Acmella oleracea possesses an anti-oxidant activity, which is supported by the findings of both in vitro and in vivo models. A majority of local community uses this plant for treating diseases like osteoporosis, cancer, cardiovascular disease, and diabetes. Our findings suggest that extracts of Acmella oleracea leaves may be a rich source of antibacterial and anticancer activity, as well as antioxidant, thrombolytic, and cytotoxic action. In addition, further study is required to examine in-vivo pharmacological (anti-oxidant, thrombolytic, cytotoxic, antibacterial) properties, as well as the causative metabolites and potential processes of Acmella oleracea leaves.
Ethical Approval and Consent
Each step was taken in accordance with the experiment's protocol, and the tests themselves were conducted after receiving clearance from a Bangladesh University ethical board by following the guidance of Helsinki – Ethical Principals. The samples were collected from willing volunteers after obtaining their informed consent.
Abbreviations
Mm, Millimeter; Conc., Concentrated; Gm, Gram; Gr, Group; HCl, Hydrochloric Acid; Hr, Hour; Min, Minute; Ml, Milliliter; μg/ml, Micro gram per milliliter; Wt, Weigh; SRA, Sajidur Rahman Akash; MMA, Md. Mahbubol Alam.
Source of Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.