Introduction
Members of Enterobacteriaceae are a group of non-sporing, non-acid fast, Gram-negative bacilli that are found in gut of humans and animals. They belong to a complex family that exhibit general morphological and biochemical similarities.1 They are the common pathogens encountered in the community and health care associated infections.2 In case of severe infections with Enterobacteriaceae, Carbapenems, were the main stay of treatment.3 Due to their unique structure and wide spectrum of activity, they were suggested as the final choice of drug for treating ESBLs and AmpC producers.4 Unfortunately, in the past few years, Carbapenem resistance among Enterobacteriaceae is one of the foremost challenges that the medical world is facing.5 Centre for disease control and prevention (CDC) classifies Carbapenem resistant Enterobacteriaceae (CRE) as an urgent threat to public health.6 CDC also defines it as any member of the family Enterobacteriaceae resistant to carbapenems like meropenem, imipenem, ertapenem or doripenem.7 Carbapenemase producing organisms are also resistant to other beta lactam antibiotics thereby leaving a very limited treatment option like tigecycline and polymyxins.8
Klebsiella pneumoniae Carbapenemases (KPC) was first identified in the United States of America in the year 2000. The presence of New Delhi metallo beta lactamases was demonstrated in United Kingdom from the clinical isolate of E coli and Klebsiella pneumoniae in a Swedish patient who had travelled India.9
Carbapenem resistance in bacteria is brought about by mechanisms like changes in outer membrane proteins over expression of efflux pumps and by carbapenem hydrolyzing enzymes.10
The mobile genetic elements carry the drug resistant genes and hence they can easily transmit from person to person via the healthcare personnel hands or through contaminated medical equipments. High level of resistance to Carbapenem and many other antimicrobial agents (fluoroquinolones and aminoglycosides) is caused by these genes.11
Early detection can prevent the spread of Carbapenemases. Hence, this study was conducted to detect the Carbapenem Resistant Enterobacteriaceae in our hospital and to evaluate a cost-effective method for carbapenem production detection.
Aim
The aim of the present research is to determine the proportion of Carbapenem resistant Enterobacteriaceae from various clinical samples received in the Department of Microbiology, MIMS Mandya for Culture and sensitivity by using Meropenem and Imipenem disk.
Materials and Methods
Inclusion criteria
All Enterobacteriaceae isolates obtained from clinical samples received for culture and sensitivity in the Department of Microbiology
Data regarding demography, culture findings and antibiotic susceptibility pattern will be collected from the laboratory record maintained in the department of Microbiology.
Data will be entered in excel sheet and analyzed for descriptive statistics like percentage.
Methodology
A total of 1624 clinical specimens received at the laboratory over a period of 6 months were included for study purposes. Clinical specimens were sputum, pus, urine, cerebrospinal fluid, body fluids like ascitic fluid, pleural fluid and others.
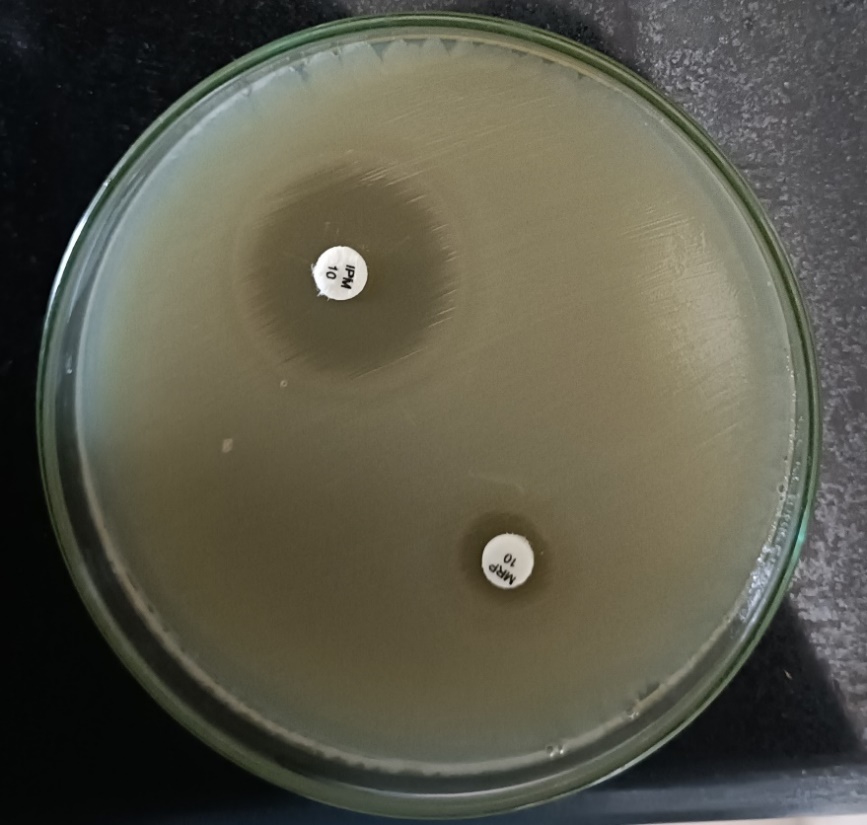
Processing of the specimens was done on MacConkey agar, Blood agar as per standard methods and incubated overnight at 37ºC. Isolated colonies were identified by using standard laboratory methods.12 Antimicrobial susceptibility testing was performed on Muller Hinton agar by Kirby Bauer disc diffusion method as recommended by the Clinical Laboratory Standards Institute (CLSI) guidelines.13 Organisms showing resistance to any one of the Carbapenem drugs including Meropenem(10µg) and Imipenem(10µg) with the susceptibility zones of </=23mm were identified as carbapenem resistant.
Results
Among 1624 clinical specimens 566 urine, 514 pus, 362 sputum, 182 body fluids were received, 211 isolates were identified as members of Enterobacteriaceae family. 50 out of 211 isolates were confirmed as Carbapenem resistant giving a prevalence rate of 23.69%. Male predominance (58%) was seen. Among 211 isolates belonging to Enterobacteriaceae family, Klebsiella pneumoniae 66 (31.27%) was the predominant organism isolated followed by Klebsiella oxytoca 52 (24.64%), Escherichia coli 49 (23.22%), Citrobacter species 32(15.16%) and Enterobacter species 12(5.68%). Among these 211 isolates, 50 were CRE, where Escherichia coli (54%) was the predominant organism isolated followed by Klebsiella pneumoniae (20%).
Table 1
Distributionof CRE in various clinical samples
|
Sample |
Number(n=50) |
Percentage |
|
Urine |
21 |
42% |
|
Pus |
17 |
34% |
|
Sputum |
12 |
24% |
|
Body Fluid |
0 |
0 |
From various samples tested CRE was predominant in urine 21(42%) followed by pus sample 17(34%), sputum 12(24%).
The prevalence of CRE was more in males (58%) compared to females (42%).
Table 3
Distribution of CRE among different species
|
Species |
Number of Isolates (n=50) |
Percentage (%) |
|
Escherichia coli |
27 |
54 |
|
Klebsiella pneumoniae |
10 |
20 |
|
Klebsiella oxytoca |
9 |
18 |
|
Citrobacter species |
3 |
6 |
|
Enterobacter species |
1 |
2 |
Among CRE, Escherichia coli (27) was the predominant organism isolated.
Table 4
Antimicrobial susceptibility pattern of CRE strains(n=50)
Among 50 CRE, all isolates were resistant towards Meropenem whereas 47 (94%) isolates were resistant towards Imipenem.
CRE strains showed high level resistance towards Fluoroquinolones, Aminoglycosides and Cephalosporins. 100% sensitivity was shown towards Colistin and Tigecycline.
Discussion
Table 5
Prevalence of CRE in various studies
|
Author |
Percentage (%) |
|
Pawar SK et al10 |
31.77 |
|
Srivastava P et al14 |
29.69 |
|
Present study |
23.69 |
The prevalence of CRE in our study is 23.69% (50/211). Pawar SK et al10 found a rate of 31.77% in Western hospital during the year 2016-2018. While a study conducted by Srivastava P et al14 found CRE prevalence rate of 29.35% from a study conducted in Uttar Pradesh.
In our study, male (58%) predominance was observed. Similar male predominance was seen in other studies, where Thomas N et al5 showed the prevalence of 53.75% in males. Pawar SK et al10 showed 65.3% prevalence in males.
In the present study CRE isolates were predominantly obtained from urine (42%), followed by Pus (34%), sputum (24%). Similar findings were obtained from Nair et al,15 where 46% of the isolates were isolated from urine samples. Srivastava P et al14 also observed that maximum number of isolates were obtained from urine samples (58.86%).
In our study Escherichia coli (54%) was the predominant organism, followed by Klebsiella pneumoniae (20%). Similar findings were observed in a study conducted by Parimala et al,16 where Escherichia coli (63.04%) was the predominant organism isolated. Srivastava P et al14 also observed that Escherichia coli was the predominant organism isolated 68.13%. The predominance of Escherichia coli could be due to the increased urine samples and Escherichia coli being a major pathogen in the urinary tract infection.17
Major part of the gut flora is contributed by Enterobacteriaceae. They also serve as reservoirs for spreading infections or contaminating the environment and fomites, especially in healthcare settings. Disinfection measures need to be followed to control the spread. Appropriate use of carbapenems will also prevent selecting resistant bacteria in a geographical area. 7
For treating invasive and life-threatening conditions, carbapenems are preferred, due to their wide spectrum of activity and concentration independent killing of bacteria. Currently Carbapenem Resistant Enterobacteriaceae infections are one of the major challenges the health care setting is facing due to its limited treatment options. 8 Hence this study was conducted to assess the prevalence rate of CRE in our hospital.
Conclusion
The high CRE prevalence rate of 23.69% suggests a major public health issue. This emphasizes the need for control of CRE spread in the community. Early identification and isolation of CRE patients with infection control practices and a strict implementation of antimicrobial stewardship programme with restricted use of carbapenems are of paramount importance in view of prevention of further increase in carbapenem resistance.