- Visibility 205 Views
- Downloads 28 Downloads
- DOI 10.18231/j.ijmr.2023.026
-
CrossMark
- Citation
A study on siderophore production and biofilm formation of Klebsiella isolated from urine samples and detection of antibiotic resistance mechanisms
Introduction
Rise in antibiotic resistance has definitely had its implications in UTI, contributing to treatment failure. Worth mentioning are the plasmid mediated extended-spectrum β-lactamases ESBLs (resistance to third generation cephalosporins) mainly in E.coli and K.pneumoniae. Other plasmid mediated resistance mechanism is AmpC enzymes among other Enterobacteriaceae (resistant to 3rd generation cephalosporins and beta lactamase inhibitors.[1]
Emergence of ESBL (extended spectrum beta lactamases) producing and Carbapenem resistant Klebsiella strains have limited the therapeutic options.
Only limited data is available on study of Klebsiella isolates from Urine samples. Hence, this study was designed to know the occurrence, detection of predominant Klebsiella species, resistance mechanisms and optimum antibiotic susceptibility pattern so that it would help the clinicians for empirical choice of antibiotics.
Several virulence factors mediate infectivity of Klebsiella, and studying them will help in further decoding the pathogenesis of Klebsiella at molecular level.[2]
Siderophores are non-redundant virulence factors used to enhance the pathogenesis of bacteria, they enhance competition among bacteria, modulate host cellular pathways, and determine the bacterial "replicative niche" during infection.[3] They mediate cellular iron homeostasis, promote dissemination of bacteria, and regulate the production of multiple bacterial virulence factors.[4]
Biofilm formation of gram-negative bacteria greatly influences the effectiveness of antibiotic therapy, because as widely studied, biofilm formation greatly deters the penetration of drugs also contributes to the resistance mechanisms of the bacteria. Hence, the need to study further to detect biofilm formation and find ways to prevent its growth. These steps will help in prevention of propagation of resistant strains and also prevent relapse after treatment.
Materials and Methods
All the Klebsiella isolated from urine samples received in the department of Microbiology laboratory at ESIC-MC and PGIMSR Bengaluru. From January 2020 to June 2021.
Sample size
Based on hospital data the proportion of Klebsiella species isolated from urine samples with significant bacteriuria calculated Sample size 184.
Inclusion criteria
Urine samples showing growth of Klebsiella pneumoniae with significant colony count (≥105 cfu/ml).
Exclusion criteria
Klebsiella species isolated from clinical specimens other than urine.
Urine samples showing mixed or insignificant growth.
Methods of detection of antibiotic resistance
Phenotypic confirmatory disc diffusion test (PCDDT) for ESBL detection
A Mueller Hinton agar plate is lawned with an overnight incubated broth of required organism to be tested. Two antibiotic discs are used a Ceftazidime disc and a combination of clavulanic acid (10 mcg) and ceftazidime (30 mcg). They are placed at a distance of 15mm. The plates are incubated for 24 hrs at 37 C. The zone diameters of both discs are measured and a difference in zone diameter of more than 5 mm for Ceftazidime- Clavulanic acid disc from Ceftazidime was considered as positive ESBL production.[5]
Amp C disk test
Lawn the surface of MHA plate with 0.5 McFarland bacterial suspension of E. coli ATCC 25922. On the surface of agar plate place a 30 μg cefoxitin disc, almost touching the disk place AmpC disk (rehydrated with 20 μl of saline) several colonies of each test organism to be applied on to the surface of Amp C disc where it touches the agar plate. Both discs should be kept almost touching each other. Invert the plate and incubate overnight at 35°C. After overnight incubation, check the plate for an indentation or a flattening of the zone of inhibition. If there is any indentation of zone of inhibition indicates that Cefoxitin was inactivated by the Amp C enzymes released by the test organism (positive result). If no indentation of zone of inhibition, indicates cefoxitin has not been inactivated, i.e. no Amp C production, (negative result).[6]
Modified Carba NP test
Inoculum was prepared by inoculating test strain in 200 μl peptone water for 2 hours (pH 7). To prepare solution A Clinical Laboratory Reagent Water phenol red (0.05%) and ZnSO4.7H2O (0.1 mmol/L) was added; pH was adjusted to 7.8 ± 0.1. Solution was stored in amber-colored bottles at 4°C for up to 15 days. The solution B was prepared by adding 12 mg/ml imipenem-cilastatin injectable form to solution A and should be stored at 4°C till it is used. Bacterial lysate (100 μl) was divided into two parts and added to two microcentrifuge tubes labeled a and b. Reagents A and B were added to tubes a and b, respectively. Both tubes incubate at 37°C and readings were taken at intervals of 10 min, 30 min, and 120 min by three different observers. Tube (a)was red and tube (b) was orange/yellow test was considered positive. Both tubes remained red test is considered negative. QC Klebsiella pneumoniae ATCC BAA 1705 (positive control), K.pneumoniae ATCC BAA 1706 (negative control), and plain A and B reagents with lysis buffer (reagent control).[7]
Biofilm detection
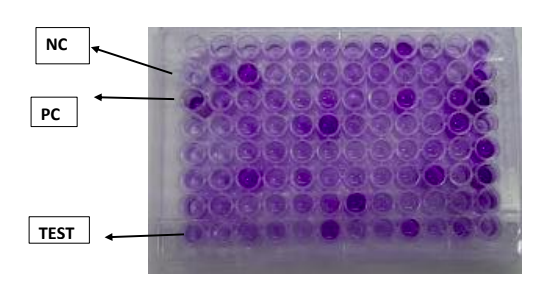
Fresh Luria-Bertani (LB) broth 100 μl is fill in each well of a 96-well polystyrene plate. Inoculate 1 μl of an overnight culture into each well. Incubate for 5 hours at 37°C. For staining of bacteria add 25 μl of 0.5% crystal violet for 20 min to each well. Discard the supernatant and wash the plates three times with deionized water to remove unbound cells. The biofilm-bound dye is then eluted with 95% ethanol, and the optical density at 550 nm (OD550) was determined. Each assay should be performed three times on at least three occasions.[8]
Characterization of isolates based on biofilm production.[9]
ODCUT = OD avg of negative control + 3(SD of ODs of negative control)
OD <=ODCUT = Non-Biofilm former (NBF)
ODCUT <OD<=2 x ODCUT = Weak biofilm former (WBF)
ODCUT <OD <4 x OD CUT = Moderate biofilm former (MBF)
OD>4x ODCUT = Strong biofilm former (SBF)
Siderophore production
Qualitative method
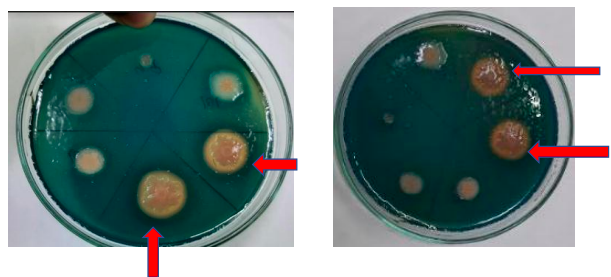
This assay was performed according to modified method given by Hu and Xu (2011). 100 ml CAS reagent is mixed in 900 ml sterilized LB agar medium to prepare CAS agar plates. Bacterial strains were inoculated by spot inoculation on each plate. After inoculation, plates were incubated for 5–7 days at 28 °C and look for the formation of orange zone around the bacterial colonies. Orange zone confirms the siderophore production by isolates.[10]
Statistical analysis
Statistical analysis was done using Epi InfoTM 7.1.4 software program. Simple frequencies were tabulated. Chi square test was done to determine the statistical significance.
Ethical consideration
Institutional ethical committee clearance was taken.
Results
Among the study population there was high preponderance of females 63.04%. Maximum number of cases were seen in the age group 21-30 age group. From study group maximum number of cases belonged to Wards (55.98%) followed by OPD (36.96%) and least number belonged to ICU(7.07%). Speciation done using usual biochemical reactions. Maximum number of isolates belonged to Klebsiella pneumoniae subsp pneumoniae (79.89%), followed by Klebsiella oxytoca (20%). Diabetes mellitus was found to be a major predisposing factor in the UTI patients, followed by presence of calculi, pregnancy in female patients also presence of invasive devices like catheter. Klebsiella species showed 80% sensitivity to Imipenem, 78% sensitivity to Meropenem, 76% sensitive to gentamicin, 74% sensitive to amikacin. Highest resistance was shown to Cefazolin, Cefotaxime & Cotrimoxazole. Out of the 184 isolates 22.8% were ESBL producers.
Out of 184 isolates, 83 were Cefoxitin resistant and these were screened by AmpC disc and 33 among 83 (39.76%) were found to be Amp C positive and, 50 were Amp C negative.
Out of the 184 isolates, 57 were found to be Carbapenem resistant isolates and they were subjected to Modified CarbaNP test. Out of the 57 only 17 (29.82%) gave positive CarbaNP test.
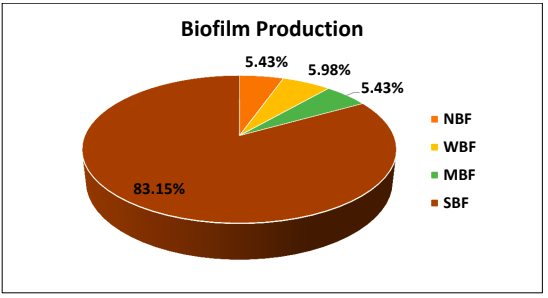
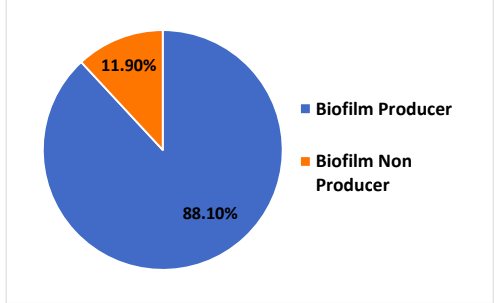
Out of the 184 isolates 83.15% were found to be strong biofilm producers, 5.43% were medium biofilm producers and 5.98% were weak biofilm producers. In total 174 out of 184 isolates were biofilm producers.
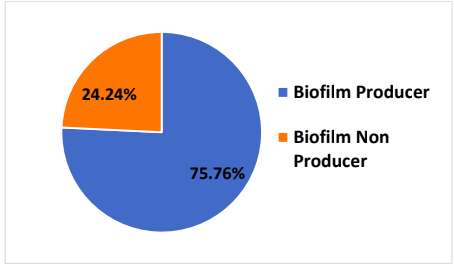
Out of the 184, 113 (61.41%) were Siderophore producers, whereas 71(38.59%) isolates were siderophore non producers.

Species wise virulence factor production
Among the 147 Klebsiella pneumoniae isolates 11 produced Siderophore alone, 58 produced biofilms alone and 78 produced both biofilm and siderophore.
|
|
Siderophore producers |
Biofilm producers |
Producing both |
|
Klebsiella pneumoniae |
11 |
58 |
78 |
|
Percentage (%) |
7.48 |
39.4 |
53.06 |
Among the 37 Klebsiella oxytoca isolates 3 produced Siderophore alone, 7 produced biofilms alone and 27 produced both biofilm and siderophore
|
|
Siderophore producers |
Biofilm producers |
Producing both |
|
Klebsiella oxytoca |
3 |
7 |
27 |
|
Percentage (%) |
8.1 |
18.91 |
72.97 |
It was shown that among the Amp C producers 75.75% were biofilm producers.
Among all ESBL producers 88% were associated with biofilm production.
|
|
Upper UTI |
Lower UTI |
|
|
Klebsiella with biofilm |
19 |
90 |
109 |
|
Klebsiella without biofilm |
3 |
4 |
7 |
|
Total |
22 |
94 |
116 |
|
|
Upper UTI |
Lower UTI |
|
|
Klebsiella with Siderophore production |
16 |
64 |
80 |
|
Klebsiella without siderophore production |
6 |
30 |
36 |
|
Total |
22 |
94 |
116 |
Discussion
Increased incidence of UTI in women when compared to men are due to anatomic differences. Relatively short urethra of female, as compared to the male counterpart, the proximity of the urethra to the anus, and the colonization of the periurethral mucosa with bowel flora are the several the reasons. Antibiotic sensitivity pattern of organisms changes rapidly over a period of time. In the present study Klebsiella species showed 80% sensitivity to Imipenem, 78% sensitivity to Meropenem, 76% sensitive to gentamicin, 74% sensitive to amikacin. Highest resistance was shown to Cefazolin & Cefotaxime.
Antibiotic resistance mechanisms
In the present study out of the 184 cases 22.8% were ESBL positive. Extended-spectrum β-lactamases (ESBLs) are a group of plasmid-mediated, diverse, complex and rapidly evolving enzymes the ability to hydrolyze third-generation cephalosporins and monobactam (aztreonam) and yet are inhibited by clavulanic acid. Carbapenems are the treatment of choice for infections due to ESBL-producing organisms. ESBL- producing organisms exhibit co-resistance to many other classes of antibiotics also, resulting in limited therapeutic options. [11]
Amp C beta lactamases
Out of 184 isolates, 83 were Cefoxitin resistant and these were screened by AmpC disc and 33 among 83 (39.76%) were found to be Amp C positive and, 50 were Amp C negative. AmpC β-lactamases are clinically significant, and these confer resistance to cephalosporins in the oxyimino group (cefotaxime, ceftazidime, ceftriaxone), 7-α methoxy cephalosporins (cefoxitin or cefotetan) and are not affected by available β-lactamase inhibitors (clavulanate, sulbactam, tazobactam).
Carbapenamase producer
Out of the 184, 57 were found to be Carbapenem resistant isolates and they were subjected to modified CarbaNP test. Out of the 57 only 17(9.24%) gave positive CarbaNP test. Globally, common carbapenemases in Enterobacterales include the Klebsiella pneumoniae carbapenemases (KPC), oxacillinase (OXA)-48-like β-lactamases, and metallo-β-lactamases, such as New-Delhi-metallo-β-lactamases (NDM), the Imipenemase(IPM), and Verona integron-encoded metallo-β-lactamases (VIM).[12]
Virulence factors
Biofilm production
Out of the 184 isolates 83.15% were strong biofilm producers, 5.43% were medium, 5.98% were weak biofilm producers. 5.43% were non biofilm producers. It is to be noted that in the present study Strong biofilm producers were strongly associated with Upper UTI, i.e 58.38% of upper UTI were associated with SBF. Also, patients with history of indwelling catheter in the present study were 100% associated with SBF. Bacteria attaches firmly to the uroepithelium and forms biofilm. Such bacteria are capable of invading the renal tissue causing pyelonephritis.
Biofilms can develop on urethral stents and catheters causing and propagating infection and blockage. Thus, catheter-associated UTI (CAUTI) is one of the most well know infections in community and associated with health care around the world [13] One of the most important point of Biofilm formation induces antimicrobial resistance by several mechanisms, like.
Limiting the diffusion of antibiotic through the matrix.
Transmission of resistance genes within the community.
Siderophore production
Out of the 184, 113 (61.41%) were Siderophore producers, whereas 71(38.59%) isolates were siderophore non producers. There is significant connection between resistance to fluoroquinolones and siderophores production. Ciprofloxacin- and norfloxacin-resistant enterococcal strains produced siderophores in large quantity.[14] Siderophore synthesis lead to Fluoroquinolone resistance resulting in the increased virulence that will increase the severity of the infection and the effectiveness of treatment. [15]
In the present study out of the 36.9% isolates that were Ciprofloxacin resistant, 41.5% were Siderophore producers. Finally, among the total isolates 105 were found to be co-producers of biofilm and Siderophore ie 57%.
Conclusion
As found in the present study high level of prevalence of Amp C, ESBL and Carbapenamases is a matter of concern. Determination of their prevalence is essential to formulate an effective antibiotic policy and to prevent treatment failure. For any person with a urinary tract infection Amikacin and Gentamicin could be used as empirical therapy provided their renal parameters are within normal limits. Virulence factors that were studied showed biofilm and siderophore production to be significantly associated with antibiotic resistance. These antivirulence therapies would allow us to effectively neutralize, or disarm the virulence of bacteria, and decrease the capacity of UTI pathogens to cause disease. Antivirulence therapeutics target processes that are critical for UTI pathogenesis and will help combat this infection.
Source of Funding
None.
Conflict of Interest
None.
References
- L Liu, F Li, L Xu, J Wang, M Li, J Yuan. Cyclic AMP-CRP Modulates the Cell Morphology of Klebsiella pneumoniae in High-Glucose Environment. Front Microbiol 2020. [Google Scholar] [Crossref]
- E Lenchenko, D Blumenkrants, N Sachivkina, N Shadrova, A Ibragimova. Morphological and adhesive properties of Klebsiella pneumoniae biofilms. Vet World 2020. [Google Scholar]
- H Namikawa, M Niki, M Niki, KI Oinuma, K Yamada, K Nakaie. Siderophore production as a biomarker for Klebsiella pneumoniae strains that cause sepsis: A pilot study. J Formos Med Assoc 2022. [Google Scholar]
- JL Faoagali. Klebsiella urinary tract infection. N Z Med J 1975. [Google Scholar]
- R Iqbal, N Ikram, M Shoaib, M Shoaib, M Javaid, RT Mehmood. Phenotypic cofirmatory disc diffusion test (PCDDT), double disc synergy test (DDST), E-test OS diagnostic tool for detection of extended spectrum beta lactamase (ESB L) producing Uropathogens. J Appl Biotechnol Bioeng 2017. [Google Scholar]
- G Gupta, V Tak, P Mathur. Detection of AmpC β Lactamases in Gram-negative Bacteria. J Lab Physicians 2014. [Google Scholar]
- SM Rudresh, GS Ravi, L Sunitha, SN Hajira, E Kalaiarasan, BN Harish. Simple, rapid, and cost-effective modified Carba NP test for carbapenemase detection among Gram-negative bacteria. J Lab Physicians 2017. [Google Scholar]
- S Kirmusaoglu. The Methods for Detection of Biofilm and Screening Antibiofilm Activity of Agents [Internet]. Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods 2019. [Google Scholar] [Crossref]
- S Kuineki, J Acharya, B Dhungel, S Adhikari, N Adhikari, UT Shrestha. Biofilm Formation and Phenotypic Detection of ESBL, MBL, KPC and AmpC Enzymes and Their Coexistence in Klebsiella spp. Isolated at the National Reference Laboratory, Kathmandu, Nepal. Microbiol Res 2021. [Google Scholar]
- K Karimi, O Zarei. Investigation of Antibiotic Resistance and Biofilm Formation in Clinical Isolates of Klebsiella pneumoniae. Int J Microbiol 2021. [Google Scholar] [Crossref]
- D Rawat, D Nair. Extended-spectrum β-lactamases in Gram Negative Bacteria. J Glob Infect Dis 2010. [Google Scholar]
- R Podschun, A Fischer, U Ullmann. Siderophore production of Klebsiella species isolated from different sources. Zentralbl Bakteriol 1992. [Google Scholar]
- SM Soto. Importance of Biofilms in Urinary Tract Infections: New Therapeutic Approaches. Adva Biol 2014. [Google Scholar] [Crossref]
- SA Jasim, SA Abdulrazzaq, SI Hashoosh, RO Saleh. Virulence Factors of Klebsiella pneumoniae Isolates from Iraqi Patients. Sys Rev Pharm 2020. [Google Scholar]
- Paweł Lisiecki. Antibiotic resistance and siderophore production in enterococci. Med Dosw Mikrobiol 2014. [Google Scholar]
- Introduction
- Materials and Methods
- Sample size
- Inclusion criteria
- Exclusion criteria
- Methods of detection of antibiotic resistance
- Phenotypic confirmatory disc diffusion test (PCDDT) for ESBL detection
- Amp C disk test
- Modified Carba NP test
- Biofilm detection
- Siderophore production
- Statistical analysis
- Ethical consideration
- Results
- Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
How to Cite This Article
Vancouver
S LI, Yoganand R, Rudresh SM. A study on siderophore production and biofilm formation of Klebsiella isolated from urine samples and detection of antibiotic resistance mechanisms [Internet]. Indian J Microbiol Res. 2025 [cited 2025 Sep 09];10(3):149-154. Available from: https://doi.org/10.18231/j.ijmr.2023.026
APA
S, L. I., Yoganand, R., Rudresh, S. M. (2025). A study on siderophore production and biofilm formation of Klebsiella isolated from urine samples and detection of antibiotic resistance mechanisms. Indian J Microbiol Res, 10(3), 149-154. https://doi.org/10.18231/j.ijmr.2023.026
MLA
S, Lakshmipriya I, Yoganand, Raksha, Rudresh, Shoorashetty Manohar. "A study on siderophore production and biofilm formation of Klebsiella isolated from urine samples and detection of antibiotic resistance mechanisms." Indian J Microbiol Res, vol. 10, no. 3, 2025, pp. 149-154. https://doi.org/10.18231/j.ijmr.2023.026
Chicago
S, L. I., Yoganand, R., Rudresh, S. M.. "A study on siderophore production and biofilm formation of Klebsiella isolated from urine samples and detection of antibiotic resistance mechanisms." Indian J Microbiol Res 10, no. 3 (2025): 149-154. https://doi.org/10.18231/j.ijmr.2023.026
