Introduction
Bloodstream infections (BSIs), also known as septicemia or bacteremia, occur when bacteria or other microorganisms enter the bloodstream and cause an infection.1, 2 BSIs can be caused by a wide range of pathogens, including bacteria, viruses, and fungi.1, 3 The most common bacteria genera responsible for BSIs are Klebsiella, Escherichia, and Staphylococcus.3, 4 BSIs can originate from various sources, including infections in other parts of the body such as the lungs, urinary tract, or surgical wounds.1, 4, 5 They can also occur as a result of medical procedures such as intravenous catheter insertion or from the spread of an existing infection.6 Symptoms of a bloodstream infection can vary depending on the severity and the underlying cause but often include fever, chills, low blood pressure, and general malaise. In severe cases, sepsis can develop, which is a life-threatening condition characterized by widespread inflammation throughout the body.1, 4
Diagnosis of BSIs involves blood cultures, where a sample of blood is collected and cultured to identify the presence of microorganisms.7, 8, 9 Treatment typically involves the administration of appropriate antibiotics or antifungal agents, depending on the causative organism.7, 8, 9, 10 In severe cases, hospitalization may be required, and intravenous antibiotics may be administered. Prevention of BSIs is crucial, especially in healthcare settings.1, 3, 4 Measures such as correct hand hygiene, sterile techniques during invasive techniques, and appropriate use of catheters and other medical devices can help reduce the risk of bloodstream infections.7, 8, 9, 10 Timely identification and appropriate treatment of infections in other parts of the body can also prevent the spread of infection to the bloodstream.1, 5 It's important to consult a healthcare professional for a proper diagnosis and treatment if you suspect a bloodstream infection or have any concerns about your health.
Regular surveillance of bloodstream infections (BSIs) etiology is an essential practice in healthcare settings to monitor the types of pathogens causing infections and their antibiotic resistance patterns.4, 6, 9 Surveillance helps healthcare facilities identify trends, implement appropriate infection control measures, and make informed decisions regarding antimicrobial therapy.7, 10, 11, 12
Here are some key components and methods used in the surveillance of BSIs etiology:13, 14, 15
Blood cultures: Blood cultures are the primary diagnostic tool for identifying the causative pathogens in BSIs. They involve collecting blood samples from patients suspected of having a bloodstream infection and culturing them in a laboratory. Positive blood cultures indicate the presence of microorganisms, which are then identified and tested for antibiotic susceptibility.
Laboratory identification: Once the blood culture bottle received then it was further incubated for 5 days in Automated system of BACT/ALERT 3D and if growth detected positive in the system, the isolated microorganisms are identified using various techniques such as biochemical tests, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), or molecular methods like polymerase chain reaction (PCR). Accurate identification helps determine the specific pathogens causing BSIs.
Antimicrobial susceptibility Testing: Testing the isolated pathogens for their susceptibility to various antibiotics is crucial for guiding appropriate treatment. The laboratory performs antimicrobial susceptibility testing using methods like disc diffusion, broth dilution, or automated systems. This information helps in monitoring antibiotic resistance patterns and selecting effective treatments.
Data collection: Surveillance programs collect relevant data on BSIs, including the type of pathogen, patient demographics, site of infection, associated risk factors, and antimicrobial susceptibility results. These data can be stored in electronic databases or specialized surveillance systems.
Data analysis and reporting: Analyzing the collected data helps identify trends, patterns, and changes in the etiology of BSIs. Regular reports and feedback are generated to inform healthcare providers, infection control teams, and public health authorities. This information can guide infection prevention strategies, antibiotic stewardship programs, and public health interventions.
Collaborative surveillance: Collaborative surveillance programs at regional, national, or international levels enable the comparison of data across different healthcare facilities and regions. These initiatives help identify emerging pathogens, track antimicrobial resistance trends, and facilitate the sharing of best practices.
Surveillance of BSIs etiology is an ongoing process and requires collaboration between healthcare professionals, laboratory personnel, and infection control teams. It aids in the early detection of outbreaks, implementation of targeted interventions, and the improvement of patient outcomes by ensuring appropriate antimicrobial therapy.8, 10
This paper focus on bloodstream infections and its antibiogram from a tertiary care hospital of western India.
Materials and Methods
Study population
The study included blood samples of all inpatients of the tertiary care hospital in the department of microbiology during April 2022–March 2023. In the study, cultures that yielded contaminants and mixed bacterial growths were not included.
Processing of samples
Blood samples bottles were collected from the patients as suggested the physician and earlier the administration of any antibiotic. The details of the patients were recorded in registers. Samples from neonates and adult were processed were loaded in BACT/ALERT3D Automated System. For negative samples blood culture bottle remains loaded in automated system and after 5 days sample is reported negative. For positive samples, the blood culture bottle is taken out from the system and then conventionally blood was inoculated with the sample were incubated at 37°C aerobically. The growth obtained was identified by colony morphology, gram stain and standard biochemical identification tests. Antimicrobial susceptibility testing was performed by Kirby–Bauer disk diffusion method and interpreted using clinical laboratory standard institute (CLSI) guidelines 2019. For bacteria from blood isolates following drugs were tested: Amikacin (AK), Augmentin (AG), Ampicillin-sulbactam (AS), Aztreonam (AC), Azithromycin (AZK), Cefazolin (CZ), Cefuroxime (CB), Ceftriaxone (RP), Ceftazidime (FG), Cefepime (ZX), Ciprofloxacin (RC), Chloramphenicol (CH), Gentamicin (GM), Imipenem (IM), Meropenem (MP), Nitrofurantoin (FD), Ceftriaxone-sulbactam (CL), Piperacillin-Tazobactam (PT), Trimethoprim-sulfamethoxazole (BA)) were tested. The susceptibility and resistance were interpreted as per CLSI guidelines 2019. Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853), and Enterococcus faecalis (ATCC 29212) were used as reference strains for culture and susceptibility testing.
Results
A total of 40 isolates were obtained and all were taken into the study. The age group varied from 2 days told to 82-year-old patient, and both genders were taken into the study. Acinetobacter spp., Enterococcus spp., Pseudomonas spp., Salmonella spp., E. aerogenes, E. coli, and K. pneumoniae were identified, the number of isolates is as mentioned in the Figure 1.
Figure 1
Number of isolates obtained in each species. Highest being the K. pneumoniae and least being the Enterococcus

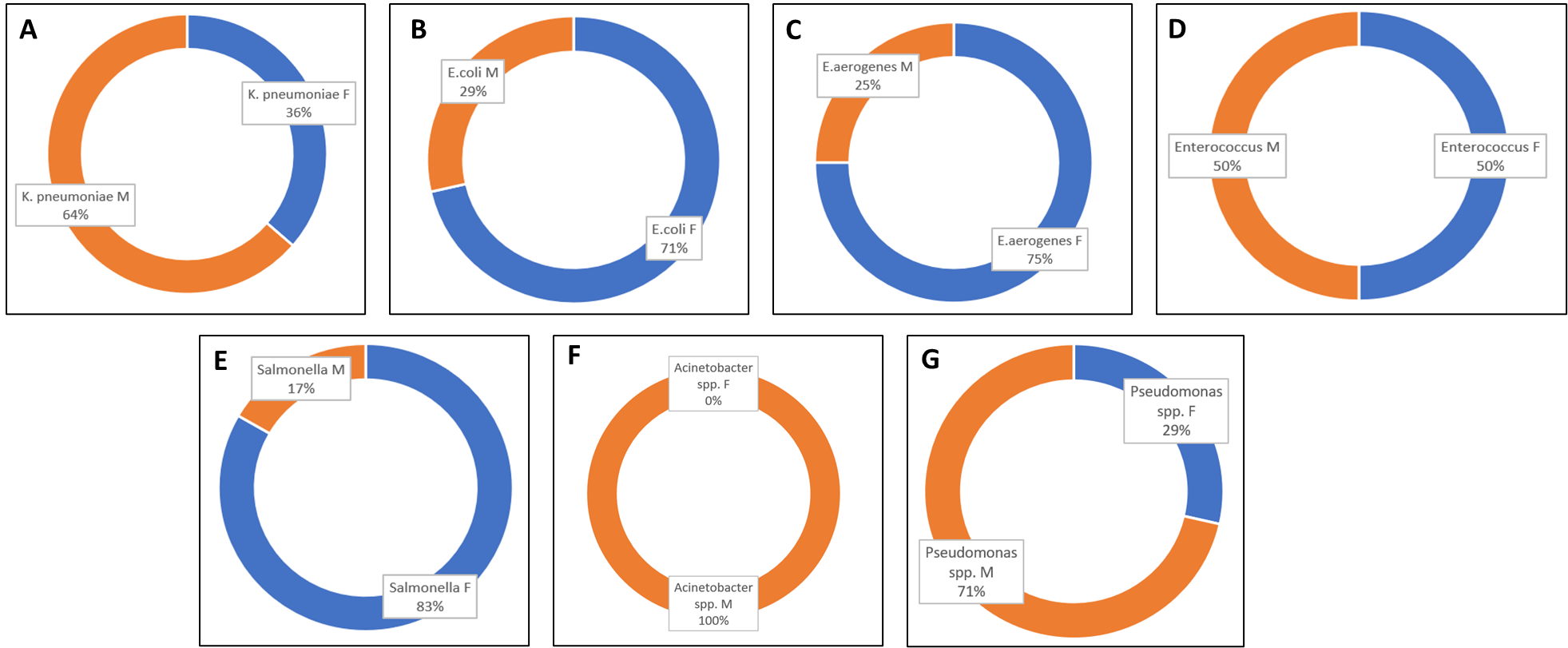
We also looked at the gender distribution (ratio to male to female) and the ratio was all most equal as shown in Figure 2.
Further, the age distribution was also seen across the enrolled patients and found all the age groups. Children age group was found to be highest in blood infection while younger adults with the least infection. The same has been shown in Figure 3.
Further the gender distribution within isolates (Figure 4), was also seen and no correlation was seen.
A heat map was obtained for following culture and antibiotic and the same has been shown in the Figure 5. Most of the isolates were susceptible as seen in the heat map.
Further, the all the isolates were plotted in bar graph for its resistance pattern against single antibiotic. Figure 6, Figure 7, Figure 8 represents the same.
Figure 5
Culture against antibiotic Heat map for K. pneumoniae, E. coli, E. aerogenes, Salmonella spp., Acinetobacter spp. and Pseudomonas spp. only

Figure 6
Susceptibility of isolates (n =38), where AK, AG, AS, AC and AZK stands for Amikacin, Augmentin, Ampicillin-sulbactam, Aztreonamand Azithromycin respectively

Figure 7
Susceptibility of isolates (n =38), Where CZ, CB, RP, FG, ZX, RC and CH stand for Cefazolin, Cefuroxime, Ceftriaxone, Ceftazidime, Cefipime, Ciprofloxacin and Chloramphenicol respectively

Figure 8
Susceptibility of isolates (n =38), where GM, IM, MP, FD, CL, PT and BA stands for Gentamicin, Imipenem, Meropenem, Nitrofurantoin, Ceftriaxone-sulbactam, Piperacillin-Tazobactam and Trimethoprim-sulfamethoxazole

Two isolates were obtained for Enterococcus species, and both of them were resistance to Penicillin-G, Gentamicin, Levofloxacin, Ciprofloxacin, Erythromycin, Linezolid, Daptomycin and sensitivity to Tetracycline. One isolate obtained from male had resistance against Vancomycin, Teicoplanin and Nitrofurantoin.
Discussion
BI are the foremost cause of illness and mortality in persons of all ages, especially in immunocompromised individuals.16 These infections are common and can result in life-threatening circumstances in hospitals.17 Bloodstream infection affects around 30 million individuals worldwide, resulting in 6 million fatalities,5 with three-million new-borns and 1.2 million children suffering from sepsis each year.18 Rapid identification, and AST of blood-borne pathogens is one of the most critical functions.10 There is a clear link between delayed medication and septic shock mortality. Each hour of wait in starting treatment is related with an 8% drop in survival.7, 14 This study aims to investigate the bacterial composition of blood culture isolates, antimicrobial trends, relate bacteraemia source.9, 12, 18
In the present study, a total of 40 isolates were isolated. Variation in culture positivity rates could be due to variance in geological setting, nature of inhabitants, epidemiological variance of the etiological causes, also volume or number of blood culture samples. The low rate of isolation in our study could be due to the fact that most of the patients would have already received antibiotic treatment at peripheral health centre before being referred to our tertiary care hospital.
Gender‑wise ratio of 1.57:1 was observed twisted in favour of males in our study in agreement with assessment done by National Hospital Discharge Survey (U.S) which states frequency of sepsis, severe sepsis, and septic shock is higher in men than in women. Infants have an extremely high attack rate, with low-birth-weight neonates being especially vulnerable. Sepsis incidence and mortality reduction after the first year of life and then rise with adding age. Furthermore, in our study, the highest positivity was found in the age group of 1 month to 12 years is 16 patients (23%) and 13 years -30 years is 9 patients (28%). In this, BSI due to Gram‑negative isolates outweighed over Gram‑positive isolates as observed in many other investigations too. Among Gram‑negative pathogens, predominance of Klebsiella species, and E. coli was seen and this is like other studies. However, nonfermenting bacilli Klebsiella spp. was more predominant. The result is of substantial worry as in the hospital setting, theses isolates are linked with a high amount of antimicrobial resistance, as seen in our study.
In present study Enterococcus isolates demonstrated higher sensitivity to vancomycin, teicoplanin, linezolid, chloramphenicol which is comparable to other Indian studies, moderately sensitive to Gentamicin and Trimethoprim‑sulfamethoxazole. High level gentamicin resistance was noted in Enterococcus isolates. In this study, most of the isolates were resistance and it was always below 10% in total.
Conclusion
Thus, present study supports the occurrence of bacterial pathogens in BSIs and their AST in our tertiary care of Western India. We can foresee the following treatment as Aminoglycoside (Amikacin, Gentamicin)/ Chloramphenicol or Piperacillin tazobactam use. Routine surveillance of BSI is needed for tracing hospital antibiograms and effective realistic treatment of BSI. Since, there are nearly zero new antimicrobials in the research pipeline, it is seen to have a careless prescription of antibiotics. Soon, we will face the issue of pan drug resistance. Implementing antibiotic consumption approaches such as restricting antibiotic use, combination therapy, antibiotic use based on antimicrobial susceptibility testing results to reduce the occurrence of BSIs and prevent resistance. Strong infection control methods and antibiotic stewardship programs are immediately needed.