- Visibility 82 Views
- Downloads 12 Downloads
- DOI 10.18231/j.ijmr.2019.047
-
CrossMark
- Citation
Infections with yeast and yeast like fungi in cancer patients with special emphasis on non- neoformans cryptococcal infections: Retrospective study
- Author Details:
-
Parijath N Goswami *
-
Ankita Kishore
Introduction
Patients with cancer are considered a population at high risk for developing invasive fungal infections. Candida spp. and Cryptococcus spp. are the yeasts most frequently isolated in clinical practice. They are important nosocomial pathogens in cancer patients and are associated with substantial morbidity and mortality, prolonged hospitalization and increased healthcare expenditures.[1],[2] Several reasons have been proposed for the increase in invasive fungal infections in cancer patients including extended survival of cancer patients as well as advances in supportive care by the use of antineoplastic and immunosuppressive agents, improved control of bacterial infections by using broad-spectrum antibiotics, hematopoietic stem cell transplantation, prosthetic devices and grafts and more aggressive surgery.[3],[4],[5]
Candida is a normal commensal of the skin, gastrointestinal and genitourinary tracts.[3] Candida sp. continue to be the most common fungal pathogens in patients with cancer. They account for 75% of total fungal infections.[6] Although Candida albicans remains the most prevalent species, there has been a clear shift towards non-albicans Candida species namely Candida tropicalis, Candida parapsilosis, Candida krusei particularly found in the neutropenic patients and Candida glabrata found especially in patients with solid tumour.[5],[7] Non- albicans Candida are of special concern, since some are highly virulent and are associated with treatment failure due to reduced susceptibilityto antifungal agents.[3] Reported increase in non- albicans Candida might have been mediated by one or more confounding risk factors in addition to selection for species that were less susceptible to azoles.[7]
The genus Cryptococcus comprises several species which are able to cause infections in human s and animals. Infections causedby them are frequently related to the exposure to avian droppings especially pigeons which are reservoirs for Cryptococcus species.[8] Cryptococcus neoformans and Cryptococcus gattii are the major pathogens within thegenus.[8] Other Cryptococcal species have traditionally been considerednon-pathogenic; however, there has been an incrementalrise in non- neoformans Cryptococcal infections namely Cryptococcus albidus, Cryptococcus laurentii, Cryptococcus luteolus, Cryptococcus uniguttulatus, Cryptococcus curvatus and Cryptococcus humicola over the past four decades.[2],[8],[9] This increase may be due to enhanced awareness of such infections, improved laboratory detection of non- neoformans Cryptococcus species, wide use of antifungals favouring the appearance of rare and more resistant species and a rise in the number of at-risk patients.[2],[8]
There are very few studies from India on the pattern of yeast infections in cancer patients. The Gujarat Cancer & Research Institute (GCRI), Ahmedabad, India is the largest cancer hospital of the country and provides state-of-the- art diagnostic and therapeutic services to the patients of all types of origin and financial background suffering from cancer. GCRI caters to a large number of patients mainly from the states of Gujarat, Rajasthan, Madhya Pradesh, Chhattisgarh and also many patients from Maharashtra, Uttar Pradesh, Bihar and Jharkhand. It is presumed that the findings of this study would faithfully reflect the pattern of infections due to yeast and yeast like fungi in cancer patients from Western India and to an extent from that of developing nations like ours.
Aims and objectives
The Aim of this Retrospective study was
To identify the different species of Candida and Cryptococcus causing infections in cancer patients,
To study their prevalence in various type of cancers and in different samples and
To find their susceptibility pattern to antifungal agents
Materials and Methods
The study was conducted retrospectively. A total of 572 samples were studied during the period of Jan 2011 to Aug 2016. Patients of both sexes and all age groups ranging from 1 to 86 years were included in the study.
The various specimens included were those routinely submitted for diagnosing the infectious agent in the Microbiology Laboratory at Gujarat Cancer & Research Institute (GCRI), Ahmedabad from patients diagnosed with cancer. Yeasts/ yeast like fungi isolated in samples received for fungal culture and also for bacterial culture were included. The various specimens from which yeasts/ yeast like fungi were isolated included Blood, Urine, Sputum, samples from Surgical Site Infections (SSI), Stool, Ascitic fluid, Bronchoalveolar Lavage (BAL), Endotracheal secretions, Pleural fluid, various Tips like central line catheter tip, Hickman catheter tip, Endotracheal tube etc. and other samples which include swabs, pus and tissue samples from sites other than SSI. Cerebrospinal fluid (CSF) samples did not show any growth of Candida sp. or Cryptococcus sp. during the study period and thus CSF has not been mentioned in this study.
Patients clinically suffering with fever, cough, expectoration and radiological findings of chest, burning micturition and symptoms of septicemia were considered to have infection. Patients on chemotheraphy, not getting treated by administration of antibiotics and still having persisting symptoms of infection were an indication for reporting yeasts. In stool, urine and sputum samples colony count of less than 1000 CFU/ ml of yeasts were considered as colonization and these isolates were not included in the study. Microscopically sputum samples having gram positive oval/ round budding yeast cells with pseudohyphal elements and patients having clinical symptoms were considered for reporting yeasts. In case of urine samples microscopic findings of having gram positive oval/ round budding yeast cells along with inflammatory cells and again patients showing clinical symptoms of urinary tract infection were considered to be infectious. Isolation of yeast in urine was considered significant when there was reproducibility of growth in two separately collected urine samples from the same patient. For stool samples the clinical history of diarrhea, nausea, Absolute Neutrophil Count (ANC) of patient and again microscopic findings were taken into consideration before reporting yeasts/ yeast like fungi. Also yeasts were reported when there was no growth of any pathogenic bacteria in these samples (Stool, urine and sputum).
Multiple episodes in the same patient were counted as separate infections unless they were caused by the same fungal agent.
Yeast/ yeast like fungi were identified on the basis of colony morphology of growth and gram stain findings. Fully automated VITEK 2 compact was used for final species identification and susceptibility testing of isolated yeasts.
Data were collected using WHONET software version 5.6 and were compiled in Microsoft excel.
Ethics statement
No informed consent was obtained from the individual patients whose data were analyzed in this non interventional study. It is not necessary to obtain approval from a medical ethics committee for this type of observational study since it contains no directly identifiable data.
Statistical analysis
The prevalence of fungal infections in various types of cancers was analyzed. The association between fungal infections in Haematological cancers and Solid tumourswas studied using Chi square test. P-value less than 0.05 was considered significant.
Results
During the study period, total1.36% (572/ 42013) samples showed infection with Candida or Cryptococcus sp. These 572 samples were studied further.
The gender ratio was 1:1 (50% each) showing no male or female preponderance.
[Figure 1] shows percentage of infections due to yeast/ yeast like fungi in different the age groups. Patients above the age of 40 years constituted 65.91%(377/ 572) of the total fungal infections, maximum i.e. 23.78% in age group 51 to 60 years followed by 21.50% in patients above 60yrs and 20.63% in 41 to 50 years of age. Minimum infection rate (4.72%) was seen in 11 to 20 years of age group patients.
[Table 1] showed prevalence of yeast and yeast like fungal infection in patients with haematological cancer to be 0.54%(129/ 23927) which is lesser than in patients with Solid tumoursi.e. 2.45%(443/18086). The result was statistically significant with a p value of less than 0.00001.
Among solid tumours the infection rate was maximum i.e. 4.01% (97/ 2416) in patients with head and neck cancers followed by 3.70%(117/ 3166) in GIT cancer patients, 2.43%(40/1649) in patients with Respiratory cancers and 1.95%(97/ 4976) in patients with Gynaecological cancers as shown in .[Table 2]
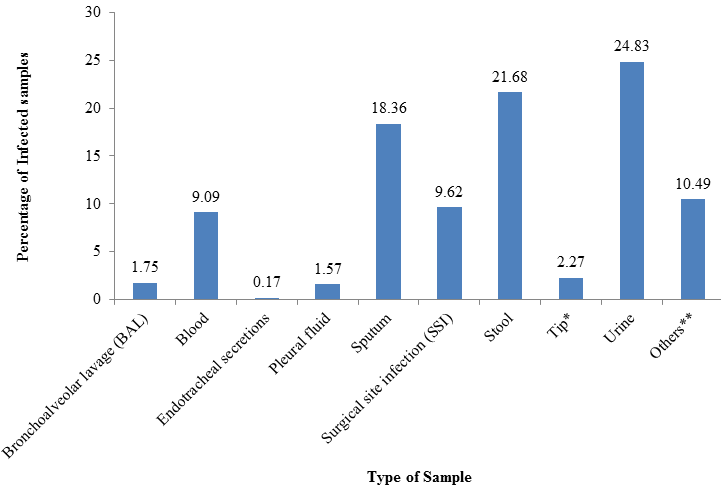
Urine (24.83%) followed by Stool (21.68%) and Sputum (18.36%) were together responsible for 64.86%(371/ 572) of the total fungal growths as shown in .[Figure 2] A total of 9.62% of Candida and Cryptococcal growths were responsible for Surgical Site Infections (SSI) while 9.09% were responsible for Blood Stream Infections (BSI). Ascitic fluid, ET secretions, Pleural fluid, BAL, Tip and other samples constituted remaining 16.43% of the growths.
[Table 3] shows that out of total 572 yeasts and yeast like fungi isolated 93.88%(537/ 572) were Candida sp. while 6.12%(35/ 572) were Cryptococcus sp. Among Candida grown more than half i.e. 63.13%(339/ 537) were non- albicans Candida sp. and 36.87% (198/537) were Candida albicans. Overall most common Candida sp.isolated was C.tropicalis i.e. 37.06% (199/ 537). Other species of Candida isolated commonly were C.glabrata (9.62%), C.parapsilosis (6.67%), C.famata (2.27%), C.krusei (2.27%) and C.guilliermondii ( 1.22%). Only 2 species of Cryptococcus were isolated. Cryptococcus laurentti constituted 85.71%(30/ 35) while Cryptococcusneoformans constituted only 14.29%(5 /35) of the total Cryptococcal growths indicating rise in non - neoformans Cryptococcal infections.
C.albicans and C.tropicalis were the two most frequent species isolated in all types of samples as listed in .[Table 4] C.tropicalis was seen more commonly in Ascitic fluid (100% - 1out of 1 isolate), Blood (38.46%), Endotracheal (ET) secretions (100% - 1out of 1isolate), Pleural fluid (44.44%) and Urine (47.89%) while C.albicans was more frequent in Bronchoalveolar lavage (BAL) (70%), Sputum (47.62%), Stool (39.52%), Tip (38.46%) and other samples (45%). Samples from Surgical Site infections (SSI) showed equal growth of both C.tropicalis and C.albicans (34.55% each). Blood samples showed growth of C.tropicalis (38.46%) most commonly followed by C.parapsilosis (34.62%), C.albicans (5.77%) and C.guilliermondii (5.77%).Ascitic fluid, BAL and ET secretions did not show any Cryptococcal growth. All the remaining samples showed growth of Cryptococcus laurentii more commonly than Cryptococcus neoformans. Out of total 30 Cryptococcus laurentii isolated 26 isolates (86.67%) were from Stool, Urine, Sputum and Blood samples.
Low level of resistance was shown by C.albicans to all the antifungal agent s tested for as shown in.[Table 5] C.tropicalis also showed low resistance with only 2.01% (4 out of 199 isolates) resistant to Fluconazole and Amphotericin B. C.parapsilosis showed no resistance to Amphotericin B and only 2.7% resistance (1 out of 37 isolates) to Fluconazole. Higher resistance rate was observed in C.krusei with 7.69% isolates resistant to Fluconazole and 15.38% resistant to Amphotericin B. Rate of resistance shown by C.glabrata to Fluconzole was also quite high i.e. 9.09%. Maximum resistance to Fluconazole was shown by C.haemulonii i.e. 66.67% (2 out of 3 isolates). Most of the Candida sp. showed good sensitivity to both Caspofungin and Micafungin except C.krusei (15.38% isolates were resistant to both the candins). Out of all the antifungal agents tested for Voriconazole was the most effective for all the yeasts isolated with highest resistance rate being 7.69% shown by C.krusei. All Cryptococcus neoformans isolated w ere sensitive to the antifungal agents tested for i.e. Amphotericin B, Flucytosine, Fluconazole and Voriconazole. Susceptibility pattern of Candida famata,Candidaspherica and Cryptococcus laurentii could not be obtained due to limitations of VITEK 2 Compact instrument.
Discussion
Fungal infections are an important cause of morbidity and mortality in cancer patients who are vulnerable to these infections.[6] Despite the limitations related to its retrospective nature, our study provides important information.
Microscopic examination gives early indication of the presence of yeasts. The observation of yeasts in normally sterile tissue or fluids is significant, provided the specimens have been collected aseptically. The possibility of yeast as a pathogen must be considered when the budding yeast cells are present and hyphae are abundant, long and thin.[10]
The prevalence of yeast/ yeast like fungi during the study period was 1.36% in our study which was comparable with a study by Paswan et al[1] which showed incidence of yeast infections to be 1.6% in patients with haematological malignancies. Another prospective study by El- Mahallawy et al[6] on fungal infection s in children with cancer show ed higher yeast infection rate i.e. 2.9%.
Candida and Cryptococcal infections were seen equally in males and females.
Patients with age more than 40 years showed 65.91% of the total yeast and yeast like fungi infections in our study probably due to more immunocompromised status in older patients. The findings were similar to a study by Hajjeh et al[7] in which 72% candedemia cases occurred among persons > 45 years old.
Higher infection rate was seen in patients with Head and neck cancers, Gastrointestinal tract cancers, Respiratory cancers and Gynaecological cancers in our study as yeasts occur as normal flora in oral cavity, skin, lower genitourinary tract and gastrointestinal tract.[3] When the immunity becomes low these commensals may cause infection. Infection rate among patients with haematologic malignancies was only 0.54% in our study. A study by L. Pagano et al[10] showed higher rate of infection (1.6%) due to yeasts in patients with haematologic malignancies. The main risk for fungal infections in patients with haematological malignancies is neutropenia. This neutropenia results from intensive cytotoxic chemotherapy and radiotherapy to totally ablate malignant bone marrow stem cells and haematopoetic stem cell transplantation resulting in graft vs host reaction.[11],[12]
Urine, stool and sputum samples were together responsible for 64.86% of total growths in this study which is again because of yeasts occurring as commensals at these sites. A study by Timothy et al[3] gave similar results with 75% of yeasts recovered from Respiratory and urine specimens. Candidiasis is the fourth common cause of nosocomial Blood Stream infections worldwide, accounting for 9% of all such infections in the United States.[1],[3] In this study blood samples were responsible for 9.09% of total growth of yeast/ yeast like fungi.
Candida sp.continue to be the most common fungal pathogen in patients with cancer.[6] In our study 93.88% yeasts recovered were Candida sp. Overall most common species of Candida isolated was Candida tropicalis (37.06%) followed by C.albicans (36.87%). This is of concern as Candida tropicalis shows a higher invasive capacity and 50 to 60% of the colonized patients develop disseminated Candidiasis.[1] In a study by Paswan et al[1] predominance of Candida tropicalis was more as compared to Candida albicans in cancer patients. Non- albicans Candida (NAC) constituted 63.13% of the total growths of Candida in this study. Other studies have also shown increasing trend towards non- albicans Candida.[1],[6],[11],[12] Prophylactic use of antifungal agents like Fluconazole are responsible for rise in NAC infections.[3],[7],[11] NACs are of special concern, since some are highly virulent and are associated with treatment failure due to reduced susceptibility to antifungal agents.[3]
Our study showed out of total 572 isolates 35 (6.12%) were Cryptococcus sp. A study by L. Pagano et al[11] on epidemiology of fungal infections in patients with haematologic malignancies showed that 8 out of 192 yeasts (4.17%) isolated were due to Cryptococcus sp. The prevalence of Cryptococcal infection increased during the Acquired Immunodeficiency Syndrome (AIDS) pandemic.[2] Out of total Cryptococcal growths 85.71% were Cryptococcus laurentii while only 14.29% were Cryptococcus neoformans in our study. A study on non- neoformans Cryptococcal infections: a Systematic Review[2] stated that 80% of non- neoformans Cryptococcal infections are due to Cryptococcus laurentii and Cryptococcus albidus. Non- neoformans cryptococcal infections have been reported rarely in humans but reports of cases of infections due to Cryptococcus laurentii havebeen increasing during the past decade.[9] This increase may be due to enhanced awareness of such infections and improved laboratory detection of these infections.[2] In addition, although the wide use of antifungals has efficiently reduced the incidence of the most prevalent pathogenic fungi, it has also favoured the appearance of niches for rare and may be more resistant species.[8] The major risk factors for non- neoformans Cryptococcal infections include impaired cell mediated immunity due to haematologic malignancy, corticosteroid therapy, organ transplantation.[2],[9] A study by D. Averbuch et al[9] showed that most of the Cryptococcus laurentii isolated were in cancer patients.
C.albicans continues to be the single most common species causing candidemia in USA, however Blood Stream Infections (BSI) due to non- albicans Candida species are increasing.[1],[7] A programme of epidemiology and fungal susceptibility performed in the USA, Canada and South America, called SENTRY13 demonstrated Candida glabrata to be the second most frequent species (first being C.albicans ) causing Candidaemia followed by C.parapsilosis. A study from Barcelona, Spain[13] showed C.parapsilosis to be the most common species responsible for BSI after C.albicans. In our study C.tropicalis (38.46%) was the most common species isolated from blood followed by Candida parapsilosis (34.62%) while Candida albicans was responsible for only 5.77% of the total BSI. Another study from India by Paswanet al1 also showed predominant isolation of C.tropicalis (49%) from blood. C.tropicalis was also the species most commonly isolated from other sterile body fluids like Urine, Pleural fluid and Ascitic fluid in our study. Other samples showed C.albicans to be the most common yeast isolated. A study by Keihn et al[12] showed that C. albicans was the most frequent isolate from all the samples including Blood, urine, Pleural fluid etc. followed by C.tropicalis and C.glabrata. In the early 1990s, increasing use of fluconazole to treat HIV-infected patients with recurrent oropharyngeal candidiasis resulted in the selection of Candida species intrinsically less susceptible to azoles and is responsible for emergence of less susceptible non- albicans Candida species.[7],[14]
In this study growth of Cryptococcus laurentii was seen more commonly than Cryptococcus neoformans in all the samples showing Cryptococcal growths. Stool, Urine, Sputum and Blood samples together showed 86.87% of the total Cryptococcus laurentii growths. The major reservoir of Cryptococcus sp. is droppings of pigeons and other birds.[8] The natural habitat of Cryptococcus laurentii is unknown.[15] However, a study by Mattson et al[16] showed feral pigeons to be carriers of medically significant fungi like Cryptococcus laurentii and Cryptococcus uniguttulatus. The ubiquitous presence of pigeons in our hospital may be a possible source of infection by Cryptococcus laurentii in our study. Infection is usually acquired by inhalation. After the initial pulmonary infection, Cryptococcus laurentii may spread to other organ systems, particularly in immunosuppressed patients even if pulmonary infection is asymptomatic.[15] Also t he presence of invasive devices have been shown to be a significant risk factor associated with Cryptococcus laurentii infection.[2],[15] Thus Blood Stream Infections due to Cryptococcus laurentii may be acquired via the intravenous catheters in our study. Another route of transmission may be nosocomial spread of infection. Cerebrospinal fluid (CSF) have been considered to be an important sample for isolation of fungal infections especially Cryptococcus sp.[17] But in our study CSF did not show any growth of yeast or yeast like fungi during the study period.
Low rates of resistance among C. albicans to various antifungal agents including Fluconazole were seen in our study. These findings have important implications for the management of C.albicans infections as Fluconazole is commonly used for treatment of uncomplicated Candida infections.[7] A low level of Fluconazole resistance was found among C.tropicalis and C.parapsilosis isolates, and a high level of resistance was detected among C.krusei isolates in our study which was consistent with other studies.[18],[13] Another finding in our study was C.glabrata showing 9.09% resistance to Fluconazole which was even higher than that shown by C.krusei (7.69%). C.krusei is considered resistant to Fluconazole but resistance pattern of C.glabrata to Fluconazole is variable.[14] High resistance to Amphotericin B was shown by C.krusei and C.guillermondii in our study. C.krusei showed high resistance to other drugs also including Caspofungin, Micafungin and Voriconazole but showed 100% sensitivity to Flucytosine. Also Voriconazole showed least in vitro resistance to most of the Candida species in our study. Voriconazole has a broad spectrum of activity and is available both orally and parenterally, and may be suitable as second-line therapy in selected patients resistant to first line agents like Fluconazole.[3]
The 5 isolates Cryptococcus neoformans were 100% sensitive to all the antifungal agents tested for i.e. Amphotericin B, Flucytosine, Fluconazole and Voriconazole in this study. Candinsi. e. Caspofungin and Micafungin are inactive against Cryptococcus species due to greater proportion of (1, 3)-α-D glucan linkages present in cell wall polymers of Cryptococcus sp. against which the candins act.[8] Therefore susceptibility tests of Cryptococcus neoformans was not performed to Candins. Susceptibility pattern of Cryptococcus laurentii could not be obtained due to limitations of our instrument – VITEK 2 Compact. There are a few studies stating that there is a favourable response of Cryptococcus laurentii to appropriate antifungal therapy but different degrees of susceptibilities to antifungal agents are seen in vitro.[9],[15]
| Diagnosis | No. of samples Infected | No. of samples Not Infected | Percentage |
| Hematological Cancers | 129 | 23798 | 0.54 |
| Solid Tumors | 443 | 17643 | 2.45 |
| Total | 572 | 41441 | 1.36 |
| Diagnosis | No. of samples received | No. of infected samples | Percentage |
| i. CNS Tumour | 1719 | 27 | 1.57 |
| ii. GIT cancer | 3166 | 117 | 3.70 |
| iii. Gynaecological cancers | 4976 | 97 | 1.95 |
| iv. Head and neck cancers | 2416 | 97 | 4.01 |
| v. Respiratory cancers | 1649 | 40 | 2.43 |
| vi. Other solid tumours * | 4160 | 65 | 1.56 |
| Total | 18086 | 443 | 2.45 |
| Species | No. of samples | Percentage |
| Candida sp. | 537 | 93.88 |
| i. C.albicans | 198 | 36.87 |
| ii. C.famata | 13 | 2.42 |
| iii. C.glabrata | 55 | 10.24 |
| iv. C.guilliermondii | 7 | 1.30 |
| v. C.haemulonii | 3 | 0.56 |
| vi. C.kefyr | 4 | 0.74 |
| vii. C.krusei | 13 | 2.42 |
| viii. C.lipolytica | 2 | 0.37 |
| ix. C.lusitaniae | 1 | 0.19 |
| x. C.parapsilosis | 37 | 6.89 |
| xi. C.pelliculosa | 1 | 0.19 |
| xii. C.rugosa | 2 | 0.37 |
| xiii. C.spherica | 1 | 0.19 |
| xiv. C.tropicalis | 199 | 37.06 |
| xv. C.utilis | 1 | 0.19 |
| Cryptococcus sp. | 35 | 6.12 |
| i. Crypto.laurentii | 30 | 85.71 |
| ii. Crypto.neoformans | 5 | 14.29 |
| Species | Sample No. of isolates (%) | ||||||||||
| Ascitic Fluid | BAL | Blood | ET Secretions | Pleural fluid | Sputum | SSI | Stool | Tip | Urine | Others | |
| C.albicans | 0 | 7 (70) | 3 (5.77) | 0 | 2 (22.22) | 50 (47.62) | 19 (34.55) | 49 (39.52) | 5 (38.46) | 36 (25.35) | 27 (45) |
| C.famata | 0 | 0 | 0 | 0 | 0 | 2 (1.9) | 1 (1.82) | 4 (3.23) | 1 (7.69) | 5 (3.52) | 0 |
| C.glabrata | 0 | 1 (10) | 0 | 0 | 1 (11.11) | 8 (7.62) | 6 (10.91) | 16 (12.9) | 1 (7.69) | 17 (11.97) | 5 (8.33) |
| C.guilliermondii | 0 | 0 | 3 (5.77) | 0 | 0 | 0 | 0 | 2 (1.61) | 0 | 0 | 2 (3.33) |
| C.haemulonii | 0 | 0 | 1 (1.92) | 0 | 0 | 0 | 1 (1.82) | 2 (1.61) | 0 | 1 (0.7) | 0 |
| C.kefyr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 (4.03) | 0 | 1 (0.7) | 1 (1.67) |
| C.krusei | 0 | 0 | 2 (3.85) | 0 | 0 | 3 (2.86) | 1 (1.82) | 1 (0.81) | 1 (7.69) | 1 (0.7) | 0 |
| C.lipolytica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.7) | 0 |
| C.lusitaniae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.7) | 0 |
| C.parapsilosis | 0 | 0 | 18 (34.62) | 0 | 1 (11.11) | 3 (2.86) | 6 (10.91) | 0 | 1 (7.69) | 3 (2.11) | 5 (8.33) |
| C.pelliculosa | 0 | 0 | 1 (1.92) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C.rugosa | 0 | 0 | 0 | 0 | 0 | 1 (0.95) | 0 | 0 | 1 (7.69) | 0 | 0 |
| C.spherica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.81) | 0 | 0 | 0 |
| C.tropicalis | 1 (100) | 2 (20) | 20 (38.46) | 1 (100) | 4 (44.44) | 31 (29.52) | 19 (34.55) | 32 (25.81) | 2 (15.38) | 68 (47.89) | 19 (31.67) |
| C.utilis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.81) | 0 | 0 | 0 |
| Crypto.laurentii | 0 | 0 | 4 (7.69) | 0 | 1 (11.11) | 5 (4.76) | 1 (1.82) | 10 (8.06) | 1 (7.69) | 7 (4.93) | 1 (1.67) |
| Crypto.neoformans | 0 | 0 | 0 | 0 | 0 | 2 (1.9) | 1 (1.82) | 1 (0.81) | 0 | 1 (0.7) | 0 |
| Total | 1 (100) | 10 (100) | 52 (100) | 1 (100) | 9 (100) | 105 (100) | 55 (100) | 12 (100) | 13 (100) | 142 (100) | 60 (100) |
| Species (Total No. of isolates) | Antifungal agent No. of resistant isolates (%) | |||||
| Amphotericin B | Caspofungin | Flucytosine | Fluconazole | Micafungin | Voriconazole | |
| C.albicans (198) | 2 (1.01) | 5 (2.53) | 1 (0.51) | 2 (1.01) | 5 (2.53) | 3 (1.52) |
| C.glabrata (55) | 2 (3.64) | 2 (3.64) | 0 | 5 (9.09) | 7 (12.73) | 2 (3.64) |
| C.guilliermondii (7) | 2 (28.57) | 1 (14.29) | 0 | 0 | 0 | 0 |
| C.haemulonii (3) | 0 | 0 | 1 (33.33) | 2 (66.67) | 0 | 0 |
| C.kefyr (4) | 0 | 0 | 0 | 0 | 0 | 0 |
| C.krusei (13) | 2 (15.38) | 2 (15.38) | 0 | 1 (7.69) | 2 (15.38) | 1 (7.69) |
| C.lipolytica (2) | 0 | 0 | 0 | 0 | 0 | 0 |
| C.lusitaniae (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| C.parapsilosis (37) | 0 | 1 (2.7) | 6 (16.22) | 1 (2.7) | 2 (5.41) | 0 |
| C.pelliculosa (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| C.rugosa (2) | 0 | 0 | 0 | 0 | 0 | 0 |
| C.tropicalis (199) | 4 (2.01) | 2 (1.01) | 1 (0.5) | 4 (2.01) | 2 (1.01) | 3 (1.51) |
| C.utilis (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Cryptococcus neoformans (5) | 0 | - | 0 | 0 | - | 0 |
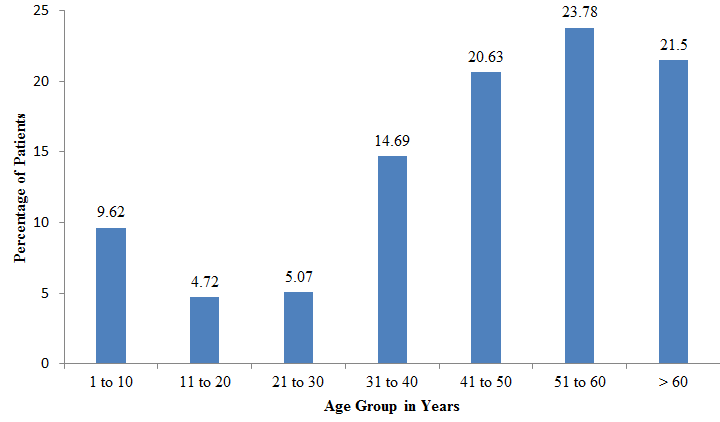
Conclusion
Patients with cancer are at particular risk for infections with yeast and yeast like fungi. With the increase in immunocompromised patients and widespread use of immunosuppressive agents non- albicans Candida and non- neoformans Cryptococcus are emerging human pathogens. The low incidence of Fluconazole resistance among isolates of C.albicans is reassuring. But the role of azole chemoprophylaxis indev elopment of drug resistance in less susceptible Candida sp. needs to be examined and new prophylaxis policies need to be made. Also non- neoformansCryptococcus are easy to miss, so a high degree of clinical suspicion, improved culture and identification techniques are required. Our findings emphasize that further studies need to be conducted to determine the antifungal susceptibility pattern of Cryptococcus laurentii.
Author’s contribution
All the authors were responsible for study conception, design, analysis and interpretation of data. The authors participated in drafting and revising the article and gave final approval for the version to be submitted.
Conflicts of Interest
All authors declare that they have no conflicts of interest with respect to the research, authorship and/or publication of this article.
Source of Funding
There are no financial conflicts to disclose. The authors have received no financial support for the research, authorship and/or publication of this article.
References
- Dinesh C.Raju, Anil K Paswan, D.K. Singh, R.K. Dubey. Isolation and distribution of Candida species among different clinical situations in critically ill patients: Prospective study. IJBR 2012. [Google Scholar]
- T Khawcharoenporn, A Apisarnthanarak, L M Mundy. Non-neoformansCryptococcal Infections: a Systematic Review.. Infection 2007. [Google Scholar]
- . D A Enoch, H A Ludlam, N M Brown. Invasive fungal infections: a review of epidemiology and management options. JMM 2006. [Google Scholar]
- Vijaya R. Bhatt, George M. Viola, Alessandra Ferrajoli. Invasive fungal infections in acute leukemia. Ther Adv Hematol 2011. [Google Scholar]
- Mukta N. Chowta, Prabha Adhikari, A. Rajeev, Ashok K. Shenoy. Study of risk factors and prevalence of invasive Candidiasis in a tertiary care hospital. Indian J Crit Care Med 2007. [Google Scholar]
- H. A. El-Mahallawy, I. Attia, N. H. Ali-El-Din, A. E. Salem, S. Abo-El-Naga. A prospective study on fungal infection in children with cancer. J Med Microbiol 2002. [Google Scholar]
- Rana A Hajjeh, Andre N Sofair, Lee H Harrison. Incidence of Bloodstream Infections Due to Candida Species and In Vitro Susceptibilities of Isolates Collected from 1998 to 2000 in a Population-Based Active Surveillance Program. J Clin Microbiol Apr 2004. [Google Scholar]
- Leticia Bernal-Martinez, Alicia Gomez-Lopez, Maria V. Castelli. Susceptibility profile of clinical isolatesof non- Cryptococcus neoformans/ non-CryptococcusgattiiCryptococcusspecies and literature review. Med Mycol 2010. [Google Scholar]
- D Averbuch, T Boekhouty, R Falk. Fungemia in a cancer patient caused by Fluconazole resistant Cryptococcus laurentii. Med Mycology 2002. [Google Scholar]
- L J R Milne. Mackie and McCartney. Pract Med Mycol . [Google Scholar]
- Morenacaira Pagano, Anna Candoni. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006. [Google Scholar]
- Timothy E Kiehn, Fitzroy F. Edwards, Donald Armstrong. The Prevalence of Yeasts in Clinical Specimens from Cancer Patients. Am J Clin Pathol 1980. [Google Scholar]
- Dolors Almirante, Benjamin J Rodriguez, - Park. Epidemiology and Predictors of Mortality in Cases of CandidaBloodstream Infection: Results from Population-Based Surveillance. J. Clin. Microbiol.Apr 2002. [Google Scholar]
- Malcolm D Richardson. Changing patterns and trends in systemic fungal infections. JAC 2005. [Google Scholar]
- P Banerjee, - Haider, - Trehan. -. Cryptococcus laurentiiFungemia. IJMM 2013. [Google Scholar]
- R Mattsson, P D Haemig, B Olsen. Feral pigeons as carriers of Cryptococcus laurentii, Cryptococcus uniguttulatus and Debaryomyceshansenii.. Med Mycol 1999. [Google Scholar]
- Parisa Badiee. Evaluation of Human Body Fluids for the Diagnosis of Fungal Infections. Bio Med Res Int 2013. [Google Scholar]
- M A Pfaller, R N Jones, G V Doern. Bloodstream Infections Due to CandidaSpecies: SENTRYAntimicrobial Surveillance Program in North Americaand Latin America, 1997-1998. Antimicrob. Agents Chemother 2000. [Google Scholar]
How to Cite This Article
Vancouver
Goswami PN, Kishore A. Infections with yeast and yeast like fungi in cancer patients with special emphasis on non- neoformans cryptococcal infections: Retrospective study [Internet]. Indian J Microbiol Res. 2025 [cited 2025 Sep 07];6(3):213-220. Available from: https://doi.org/10.18231/j.ijmr.2019.047
APA
Goswami, P. N., Kishore, A. (2025). Infections with yeast and yeast like fungi in cancer patients with special emphasis on non- neoformans cryptococcal infections: Retrospective study. Indian J Microbiol Res, 6(3), 213-220. https://doi.org/10.18231/j.ijmr.2019.047
MLA
Goswami, Parijath N, Kishore, Ankita. "Infections with yeast and yeast like fungi in cancer patients with special emphasis on non- neoformans cryptococcal infections: Retrospective study." Indian J Microbiol Res, vol. 6, no. 3, 2025, pp. 213-220. https://doi.org/10.18231/j.ijmr.2019.047
Chicago
Goswami, P. N., Kishore, A.. "Infections with yeast and yeast like fungi in cancer patients with special emphasis on non- neoformans cryptococcal infections: Retrospective study." Indian J Microbiol Res 6, no. 3 (2025): 213-220. https://doi.org/10.18231/j.ijmr.2019.047
