- Visibility 184 Views
- Downloads 34 Downloads
- DOI 10.18231/j.ijmr.2019.059
-
CrossMark
- Citation
Detection of carbapenamase production by rapid carba NP test among Enterobacteriaceae isolates in tertiary care hospital
- Author Details:
-
Kanagiri Tejasvi
-
B Anuradha *
Introduction
Multidrug resistance is the emerging problem at an alarming rate causing both Nosocomial and community acquired infections.[1] Gram negative bacteria specifically Enterobacteriacea is the most common cause of community as well as Hospital acquired infections including urinary tract infections, peritonitis, septicemia, pulmonary infections, soft tissue infections and device associated infections. As Carbapenems (imipenem, meropenem, ertapenem and doripenem ) are last line of therapy for Extended spectrum β lactamases producing organisms and most frequently required to treat Nosocomial infections, infections due to carbapenem resistant organisms have become a great concern as it leaves health care system with limited therapeutic options.
Carbapenemases are carbapenem hydrolyzing beta-lactamases that confer resistance to a broad spectrum of beta-lactam substrates including carbapenems. Resistance to carbapenems is mostly mediated by production of carbapenemases, decreased outer membrane permeability and efflux pump mechanisms.[2] Non carbapenemase related mechanism of carbapenem resistance is not transferable[2],[3],[4] whereas Carbapenemase related are transferrable through plasmid and are potentially responsible for outbreaks and are largely associated with multi or pan drug resistance in gram negative bacteria, particularly Enterobacteriaceae.[2],[3],[4] Early and rapid detection of carbapenemase producing gram negative bacteria is of utmost importance for reducing primary and secondary infections and also helps in the containment of spread of infection.
Various carbapenemases have been reported in Enterobacteriaceae such as: Klebsiella pneumonia carbapenemase (KPC; Ambler class A); Verona integron – encoded metallo -β-lactamase (VIM), imipenemase (IMP), New Delhi Metallo -β-lactamase(NDM) (all belong to Ambler class B) ; and oxacillinase-48 ( OXA – 48; Ambler class D).[2],[5],[6],[7]
Several phenotypic methods like disc diffusion, MIC determination are most widely used in routine diagnostic practices to determine carbapenem susceptibility or resistance pattern. Ertapenem is preferred over Imipenem or Meropenem for invitro susceptibility testing due to its superior sensitivity and has reported to detect most carbapenemase producers.[8],[9] Other tests for screening and detection of carbapenemases such as modified Hodge test which is not highly sensitive and specific[2],[8],[10] and molecular detection of carbapenemase genes which are time consuming,[2],[8],[11],[12] highly expensive , require expertise and well established laboratory.
Nordmann et al.[13] have developed a biochemical based test Carba NP, which is rapid can detect production of carbapenemase within 2hrs, easy to perform with good sensitivity and high specificity, inexpensive and reproducible.
Though phenotypic methods are routinely done but as they are time consuming. The present study compares the rapid Carba NP test with Ertapenem disk diffusion (DD) method for detection of carbapenemase producers among Enterobacteriaceae.
Clinical Standards Institute (CLSI) recommends Carba NP (CNP) test as confirmatory test for carbapenemase production among Enterobacteriaceae, Psuedomonas aerugi nosa and Acinetobacter species .[14]
Materials and Methods
A prospective study was conducted for a period of two months among 150 Enterobacteriaceae species (Escherichia coli 88, Klebsiella pneumonia 49 and others 13) isolated from various clinical samples (blood, urine and sputum) in teritiary care Hospital. The study was conducted after obtaining Ethical committee clearance.
Identification of these strains was done using standard phenotypic methods such as Gram’s strain, growth characteristics and biochemical reactions. Resistance to carbapenems was detected using Ertapenem (10µg) disk by Kirby Bauer disk diffusion(DD) method and Carba NP test developed by Nordmann et al and interpreted as per the CLSI standards.[13]
The chemicals, antibiotic disk and media required for performing the test were procured from Himedia Laboratories, Mumbai. Inject ion Imipenem + Cilastatin for Carba NP test was procured from Ranbaxy laboratories, Mumbai.
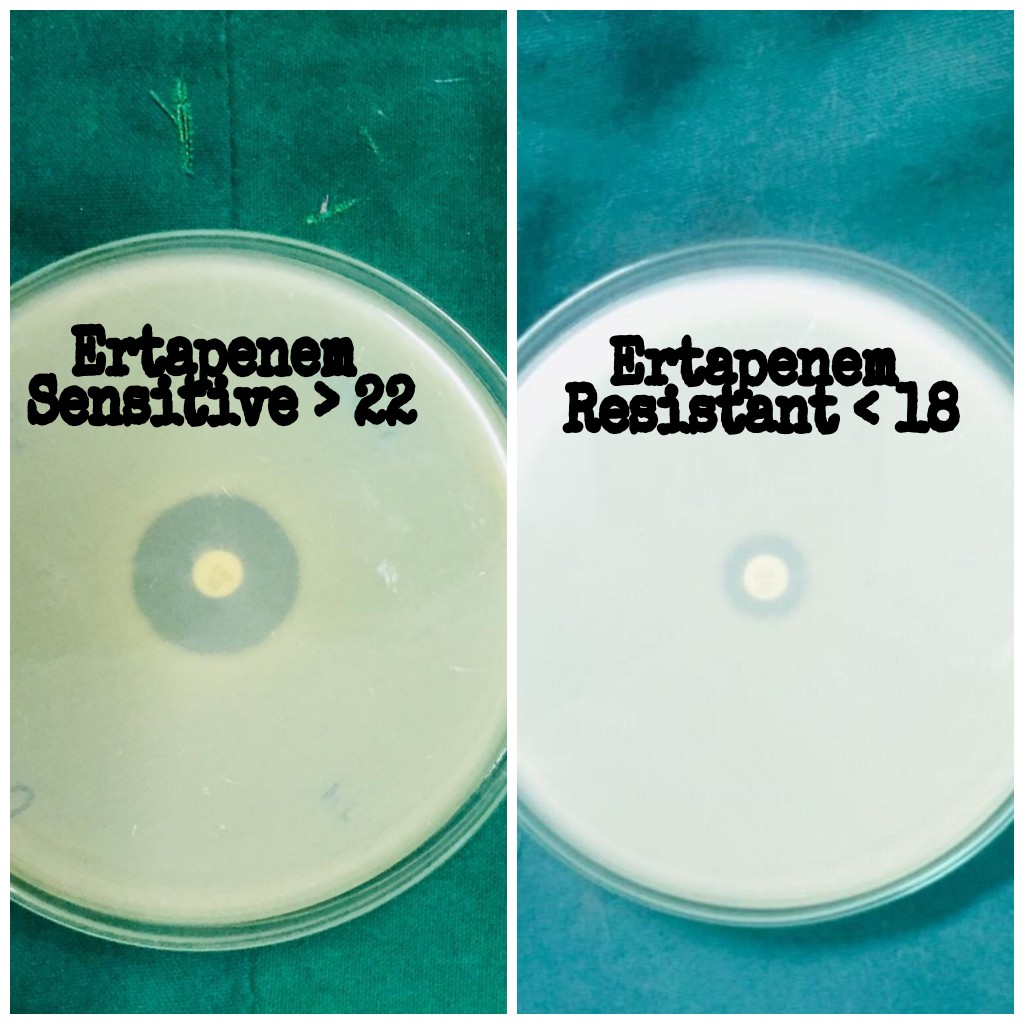
Ertapenem disk diffusion method
A lawn culture of Enterobacteriaceae isolates was done on Muller Hinton agar. 10µg of Ertapenem disk is suspended on the agar surface and incubated at 37ºC overnight and interpreted as per Clinical Standards Institute (CLSI) standards.

Carba NP test
Principle
Carba NP based on the detection of Imipenem hydrolysis by carbapenemase producing bacteria. Hydrolysis acidifies the medium which results in colour change of the pH indicator.
Reagents
Clinical laboratory reagent water, commercially available bacterial protein extract reagent in Tris HCL buffer, pH 7.4, zinc sulfate heptahydrate, phenol red powder, 1 N NaOH solution, 10% HCL solution, microcentrifuge tubes 1.5ml, 1µl inoculation loops.
Preparation of carba NP solution A
2ml of 0.5ml phenol red solution is added to 16.6ml clinical laboratory reagent water, vortexed and adjusted to a final pH 7.8 by adding NaOH. Phenol red is the pH indicator. Carbapenemase producing strain breaks down imipenem into acidic products which turns the color of the phenol red indicator to yellow. A volume of 180 µl ZnSO4 was added to obtain a final concentration of 0.1mMZnSO4. ZnSO4 is added to enhance the activity of metallo-beta-lactamase (MBL) carbapenemases which increases the sensitivity of Carba NP test to detect MBL carbapenemases.
Preparation of Carba NP solution B
Solution A + 12mg/ml imipenem cilastatin injectable form (equivalent to 6mg of imipenem reference standard powder).
Procedure
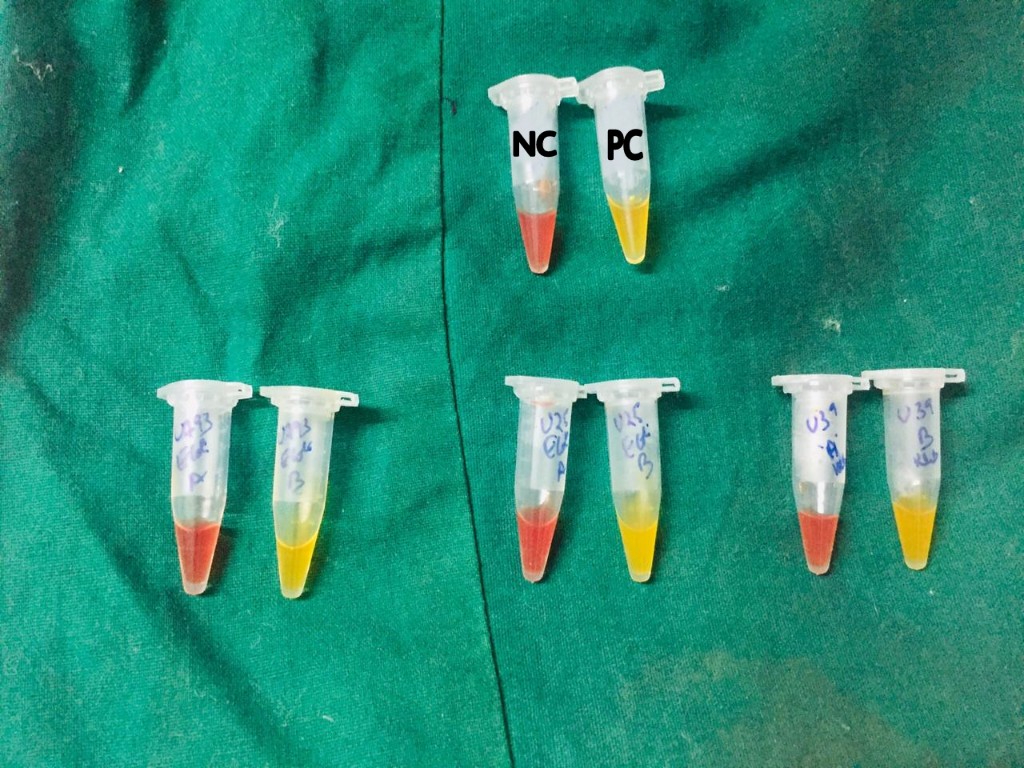
[13],[14] Bacteria grown on Muller Hinton Agar (MHA) is taken with 1µl loop and suspended in 1.5ml eppendorf tube containing 100µl of 20mMTris Hcllysis buffer and mixed using a vortex device for 5s. This lysate is then mixed with 100µl of aqueous indicator solution containing 0.05% phenol red with 0.1mmol/liter ZnSO4, previously adjusted to pH 7.8 and 6mg/ml Imipenem powder or 12mg/ml Imipenem + Cilastatin injectable form (equivalent to 6mg of Imipenem standard powder) is taken as reaction tube or tube “A” and control tube or tube “B” as indicator solution without antibiotic. Tubes are vigorously mixed for 5 to 10s initially both tubes are red or red – orange in colour, incubated at 37ºC and monitored for 2h and observed for colour change.
Interpretation
After 2h, If:
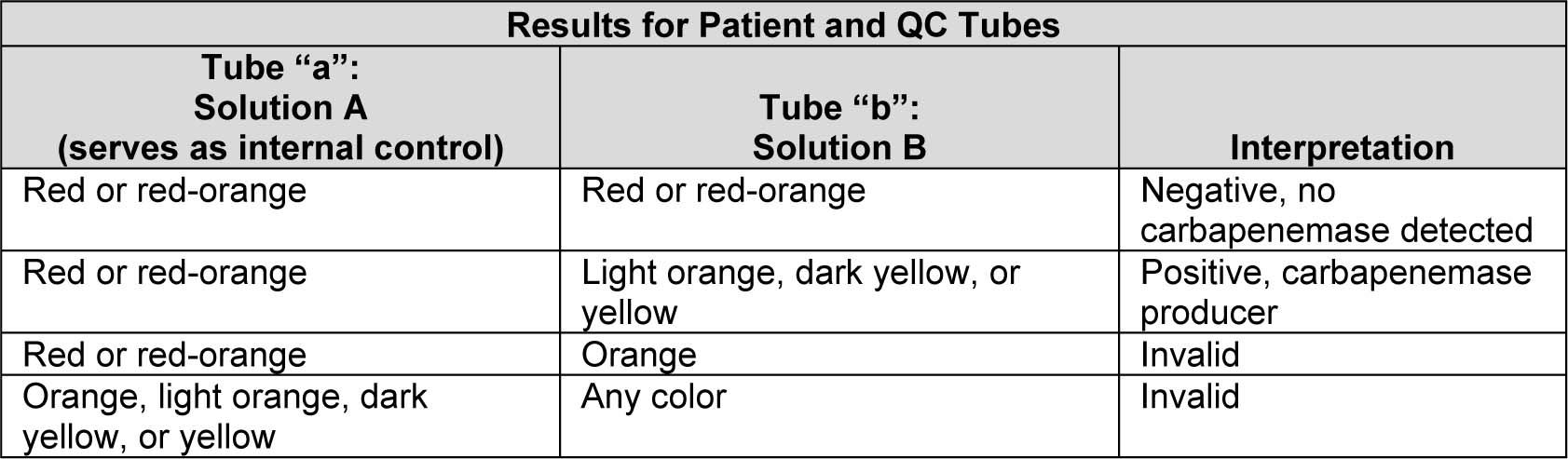
CNP positive – tested isolated is carbapenemase producer.
CNP negative – tested isolated is non carbapenemase producer.
Quality control
With every panel of test isolates, quality control strains were tested. Klebsiella pneumoniae ATCC BAA-1705 as positive control and Escherichia coli ATCC 25922 as negative control.
Statistical analysis
The collected data were analyzed using statistical package for social sciences (SPSS) software (version 21.0) and Epi – info softwares.
Results
Among 150 Enterobacteriaceae isolates, Carba NP positive were 34(22.6%) and negative were 116(77.3%). Ertapenem disk diffusion detected, 122(81.3%) as susceptible, 8(5.3%) as intermediate and 20(13.3%) as resistant.
Out of 116 Carba N P negative strains, 7 are resistant to Ertapenem. Out of 34 Carba NP positive, 13 are sensitive to Ertapenem by disk diffusion method.
Carba NP has a sensitivity (61.76%), specificity (93.97%), PPV (75%), NPV (89.34%), accuracy (86.67 %) which are statistically significant with ‘p’ value <0.05
| Number | Percentage | |
| Escherichia coli | 88 | 58.6% |
| Klebsiella pneumoniae | 49 | 32.6% |
| Others(citrobacter spp -7, proteus spp -6) | 13 | 8.6% |
| Total | 150 |
| Number | Percentage | |
| Sensitive | 122 | 81.3% |
| Intermediate | 8 | 5.3% |
| Resistant | 20 | 13.3% |
| Total | 150 |
| Positive | 34 | 22.6% |
| Negative | 116 | 77.3% |
| Total | 150 |
| Ertapenem Susceptibility | CNP Positive | CNP Negative | Total |
| Resistance | 21 | 7 | 28 |
| Sensitive | 13 | 109 | 122 |
| Total | 34 | 116 | 150 |
For Ertapenem disk diffusion both resistant and intermediate is has been considered under resistant.
Discussion
In the present study total Enterobacteriaceae isolates are 150 among them Escherichia coli- 88 (58.6%), Klebsiella pneumoniae – 49(32.6%) and others -13(8.6%). All the isolates were tested for rapid Carba NP test and Ertapenem sucpetibility by Kirby Bauer disk diffusion method. Similar study was done by Shinde et al who has tested for 400 Enterobacteriaceae among them Escherichia coli 244(61%), Klebsiella pneumoniae 135(33.75%), others 21(5.25%).
All the 150 isolates of Enterobacteriaceae were first tested for Ertapenem susceptibility by Kirby Bauer disk diffusion method and interpreted as per Clinical Laboratory Standards Institute (CLSI). Out of 150 isolates Ertapenem susceptible were found to be 122(81.3%), intermediate were 8(5.3%) and resistant were 20(13.3 %) which is compared to Shinde et al[15] who had studied for 400 isolates out of which Ertapenem susceptible are 302, intermediate are 16 and resistant are 82.
Ertapanem is preferred over Imipenem or Meropenem for invitro susceptibility testing due to its superior sensitivity (97% vs 42% and 71%)[16] and has reported to detect most carbapenemase producers as reported by Nordmann et al and Gniadkowski et al in their study.[8],[9]
All the 150 isolates of different Enterobacteriaceae species were tested for carbape nemase production by rapid Carba NP test developed by Nordmann et al. Out of 150 isolated 34(22.6%) were positive, that are carbapenemase producers and 116(77.3%) were negative, non-carbapenemase producers which were compared to Shinde et al[15] who tested Carba NP for 400 isolates, out of which 106 were positive, carbapenemase producers and 294 were negative, non carbapenemase producers.
Carba NP test developed by Nordmann et al[9] demonstrated that Carba NP has high sensitivity and high specificity when compared with the molecular studies where resistance genes can be detected. Tijet et al[17] in 2013 have also reported excellent specificity but lower sensitivity.
In the present study sensitivity and specificity of Carba NP test is 61.76% and 93.97% respectively which correlates with Tijet et al[17] in 2013 and Vasoo et al[18] who reported an excellent specificity but a lower sensitivity.
Based on the high specificity and negative predictive value of Carba NP test, In the present study 13strains which are Carba NP positive due to carbapenemase production were susceptible by disk diffusion which Ertapenem failed to detect which can lead to adverse consequences in clinical management of the patient which correlates with Shinde et al[15] were 36 strains which are Carba NP positive were falsely susceptible by Ertapenem disk diffusion method.
In another condition 7 strains which were resistant by Ertapenem disk diffusion method was found negative by Carba NP test due to carbapenem resistance other than carba penemase which correlates with Shinde et al[15] were 28 strains which were resistant by Ertapenem disk diffusion test was tested negative by Carba NP test which may be due to true negativity as a result of carbapenem resistance other than carbapenemases.
The above results indicated that Carba NP test has multiple benefits. It is rapid, cheaper, highly specific and most widely used to identify carbapenemase producers than other phenotypic tests.
In a study done by Galani et al and Habrak et al[16],[19] who has done molecular techniques like real time PCR for detection of carabapenemase gene which however cannot detect the resistance mechanism of carbapenamases and also expensive, requires expertise and well established laboratory making it unsuitable for routine purposes in the laboratories. Whereas Carba NP is easy to perform, rapid (within 2hrs), cheaper, does not require man power, requires minimal reagents and can be performed on routine basis in laboratories for detection of carbapenamse resistance.
Conclusion
Carba NP detects larger number of carbapenemases within sh orter time (<2h) compared to Ertapenem disk diffusion method (16-18h) which is rapid, gives result in single day with minimal reagents , cheaper, less man power and not labor intensive.[3] Carba NP also detects carbapenemase producers where Ertapenem fails to detect which is of clinical significance. The rise in prevalence of Carbapenemase producing strains associated with significant mortality recommends the incorporation of this simple and rapid cost effective test in routine diagnostic laboratories there by benefitting patient care and antimicrobial stewardship.
Source of Funding
None.
Conflict of Interest
None.
References
- B Spellberg, M Blaser, R J Guidos, H W Boucher, J S Bradley. Combating antimicrobial resistance : policy recommendations to save lives. Clin Infect Dis 2011. [Google Scholar]
- P Nordmann, T Naas, L Poirel. Global spread of carbapenemase producing Enterobacteriaceae. Emerg Infect Dis 2011. [Google Scholar]
- Nordmann P. Carbapenemase - producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect 2014. [Google Scholar]
- J D Pitout. Multiresistant Enterobacteriaceae: A new threat of an old problem. Exp Revi Anti-Infect Ther 2008. [Google Scholar]
- L Martinez - Martinez. Extended - spectrum --lactamases and the permeability barrier. Clin Microbiol Infect 2008. [Google Scholar]
- K S Thomson. Extended spectrum lactamases, Amp C and carbapenemase issues. J Clin Microbiol 2010. [Google Scholar]
- R C Moellering. NDM-1 - a cause for world concern. N Engl J Med 2010. [Google Scholar]
- P Nordmann, M Gniadkowski, C G Giske, L Poirel, N Woodford, V Miriagou. European Network on Carbapenemases. Indentification and screening of carbapenemase producing Enterobacteriaceae. Clin Microbiol Infect 2012. [Google Scholar]
- P Nordmann, L Poirel. Strategies for identification of carbapenemase producing Enterobacteriaceae. J Antimicrob Chemother 2013. [Google Scholar]
- F Pasteran, T Mendez, M Rapoport, L Guerriero, A Corso. Controlling false-positive results obtained with the Hodge and Masuda assays for detection of class A carbapenemase in species of Enterobacteriaceae by incorporating boronic acid. J Clin Microbiol 2010. [Google Scholar]
- . Centers of Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo--lactamases - United States. MMWR Morb Mortal Wkly Rep 2010. [Google Scholar]
- P Nordman, L Poirel, A Carrier, Toleman Ma, T R Walsh. how to detect NDM-1 producers?. J Clin Microbiol 2011. [Google Scholar]
- P Nordmann, L Poirel, L Dortet. Rapid detection of carbapenemase producing Enterobacteriaceae. Emerg Infect Dis 2012. [Google Scholar]
- . Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. 25th Informational Supplement. CLSI Document M100-S25. 2015. [Google Scholar]
- S Shinde, R Gupta, S S Raut, G Nataraj, P R Mehta. Carba NP as a simpler,rapid,cpst-effective, and a more sensitive alternative to other phenotypic tests for detection of carbapenem resistance in routine diagnostic laboratories. J Lab Physicians 2017. [Google Scholar]
- I Galani, P D Rekatsina, D Hatzaki, D Plachouras, M Souli, H Giamarellou. Evaluation of different laboratory tests for the detection of metallo beta lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008. [Google Scholar]
- N Tijet, D Boyd, S N Patel, M R Mulvey, R G Melano. Evaluation of the Carba NP test for rapid detection of carbapenemase producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013. [Google Scholar]
- S Vasoo, S A Cunningham, P C Kohner, P J Simner, J N Mandrekar, K Lolans. Comparison of a novel,rapid chromogenic biochemical assay,the carba NP test, with the modified Hodge test for detetction of carbapenemase producing Gram-negative bacilli. J Clin Microbiol 2013. [Google Scholar]
- J Hrabk, E Chudckov, C C Papagiannitsis. Detection of carbapenemases in Enterobacteriaceae: A challenge for diagnostic microbiological laboratories. Clin Microbiol Infect 2014. [Google Scholar]
How to Cite This Article
Vancouver
Tejasvi K, Anuradha B. Detection of carbapenamase production by rapid carba NP test among Enterobacteriaceae isolates in tertiary care hospital [Internet]. Indian J Microbiol Res. 2019 [cited 2025 Sep 10];6(3):272-276. Available from: https://doi.org/10.18231/j.ijmr.2019.059
APA
Tejasvi, K., Anuradha, B. (2019). Detection of carbapenamase production by rapid carba NP test among Enterobacteriaceae isolates in tertiary care hospital. Indian J Microbiol Res, 6(3), 272-276. https://doi.org/10.18231/j.ijmr.2019.059
MLA
Tejasvi, Kanagiri, Anuradha, B. "Detection of carbapenamase production by rapid carba NP test among Enterobacteriaceae isolates in tertiary care hospital." Indian J Microbiol Res, vol. 6, no. 3, 2019, pp. 272-276. https://doi.org/10.18231/j.ijmr.2019.059
Chicago
Tejasvi, K., Anuradha, B.. "Detection of carbapenamase production by rapid carba NP test among Enterobacteriaceae isolates in tertiary care hospital." Indian J Microbiol Res 6, no. 3 (2019): 272-276. https://doi.org/10.18231/j.ijmr.2019.059
