- Visibility 188 Views
- Downloads 24 Downloads
- Permissions
- DOI 10.18231/j.ijmr.2020.041
-
CrossMark
- Citation
Prevalence of extended-spectrum β-lactamase in Pseudomonas aeruginosa in tertiary care centre
- Author Details:
-
Swati Tewari
-
Alok Kumar
-
Ekta Rani *
Abstract
Introduction: Pseudomonas aeruginosa an opportunistic nosocomial pathogen, its increasing resistance to broad-spectrum beta-lactams, mediated by extended-spectrum betalactamase enzymes (ESBL), is problem worldwide. The present study was undertaken to determine the prevalence of ESBL-production among the clinical isolates of Pseudomonas aeruginosa.
Materials and Methods: Various clinical specimens received in our laboratory were processed and Pseudomonas aeruginosa was identified as per standard microbiological procedure. All isolates were subjected for ESBL screening test. Potential ESBL producer was then subjected for ESBL Phenotypic confirmatory test –Disc Diffusion method. Antimicrobial susceptibility test was performed by Kirby – Bauer disc diffusion method on all confirmed isolates as per Clinical Laboratory Standard Institute (CLSI 2016) guidelines.
Results: A total of 322 non duplicate isolates of Pseudomonas aeruginosa identified during the study period. Of these 26.09% (n = 84) of Pseudomonas aeruginosa isolates were found to be ESBL producers. All ESBL positive Pseudomonas aeruginosa isolates showed high resistance to ciprofloxacin 79 (94.05%), Gentamicin 61 (72.62%) and tobramycin 60 (71.43%). Resistance was low to drugs like cefoparazone + salbactum 17 (20.24%) and piperacillin + Tazobactum 14 (16.67%), and Imipenem 15 (17.86%). All the isolates showed 100% sensitive to Polymyxin B.
Conclusion: Present study highlights the prevalence and drug resistance of ESBL positive Pseudomonas aeruginosa. Regular antimicrobial susceptibility monitoring is essential for judicial use of antibiotics in order to prevent the spread of drug resistance.
Introduction
Pseudomonas aeruginosa well known opportunistic pathogen, and causes life threatening nosocomial infections due to its intrinsic resistance to many antibiotics and development of increased resistance in healthcare settings. The increasing resistance of potentially pathogenic bacteria to multiple conventional antibiotics is an urgent problem in global public health.[1] Among various mechanisms of resistance, ß-lactamase production is the most important, resistance mechanism among P. aeruginosa. Under these ß-lactamases, ESBL producing P. aeruginosa continues to pose a challenge to infection control worldwide. Extended-spectrum ß-lactamases (ESBLs) belong mostly to class A of the Ambler classification scheme and group 2be according to the Bush-Jacoby-Medeiros functional scheme.[2], [3]
The 2 be designation consists of ‘2b’ denoting that the enzyme is derived from a 2b enzyme (e.g., SHV-1, TEM-1, and TEM-2) and ‘e’ representing the extended spectrum of activity, ESBL producing gram negatives are typically resistant to Penicillin, first and second generation cephalosporins and also third generation oxyimino cephalosporins and monobactams. ESBLs in P. aeruginosa includes the, SHV, TEM, PER, VEB and IBS/GES types[4] Early and accurate detection of ESBL producing P. aeruginosa is important for optimal treatment of critically ill and hospitalized patients and also, to control the spread of resistance. Hence, the present study was conducted with an objective to find the prevalence of ESBL producing P. aeruginosa and also to serve as a guide for doctors managing patients by implementing proper infection control measures as well as formulating an effective antibiotic strategy.
Materials and Methods
The present study was conducted in the Department of Microbiology at Muzaffarnagar Medical College, Muzaffarnagar, over a period from January 2014 to July 2015. All 322 isolates of Pseudomonas aeruginosa obtained from various clinical samples received in microbiology laboratory from IPD & OPD were included in the study. The isolates were identified as per the standard microbiological procedures.[5] Antimicrobial sensitivity testing was performed on Mueller-Hinton agar plates with commercially available disks (Himedia) by Kirby Bauer disk diffusion method and interpreted as per CLSI guidelines.[6]
The results of susceptibility test were divided into susceptible and resistant. The isolates with intermediate susceptibility were included in resistant category.
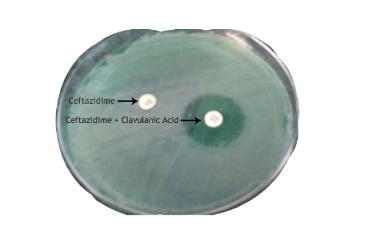
Isolates were considered a potential ESBL producer if the zone of inhibition for ceftazidime was observed to be <22mm. Potential ESBL producer was then subjected for ESBL Phenotypic confirmatory test –Disc Diffusion method as recommended by Clinical Laboratory Standards Institute, 2015.[7]
Results
A total of 322 non duplicate isolates of Pseudomonas aeruginosa identified during the study period. Out of 322 isolates, 226 (70.19%) isolates of Pseudomonas aeruginosa showed zone of inhibition ≤ 22 mm for third generation cephalosporin, Ceftazidime. of these 26.09% (n = 84) of Pseudomonas aeruginosa isolates were found to be ESBL producers[[Figure 1]].
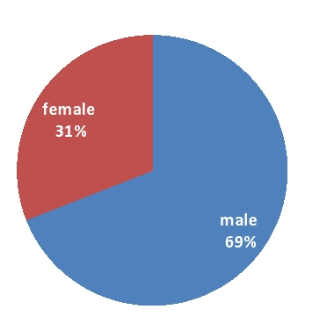
Prevalence of ESBL producing Pseudomonas aeruginosa (84/322) in this study was 26.09%. Out of which 58(69.05%) samples were of male patients and 26(30.95%) were from female patients[[Figure 2]].
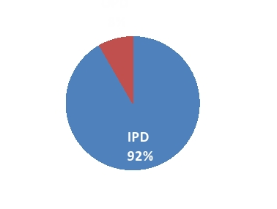
In Patient Department (IPD) 77 (91.67%) and only 07 (8.33%) samples were of OPD patients[[Figure 3]].
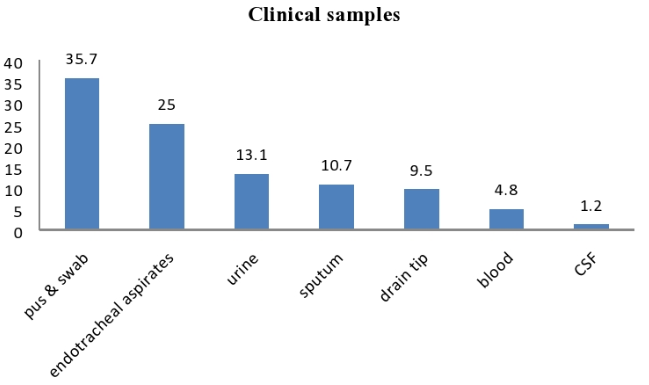
We isolated ESBL positive P. aeruginosa from different type of samples, out of which maximum number were of pus and swabs 30 (35.7%) followed by Endotracheal aspirates 21 (25%), urine 11(13.10%), sputum 09 (10.71%), drain tip 08 (9.52%), blood 04 (4.76%) & cerebrospinal fluid 01 (1.19%)[[Figure 4]].
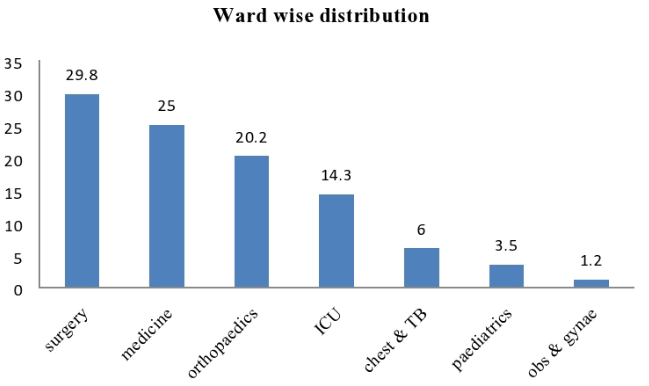
Out of 84 ESBL positive samples of pseudomonas aeruginosa maximum sample received from Surgery 25 (29.76%) followed by Medicine 21 (25%), Orthopaedics 17 (20.24%), ICU 12 (14.29%), Chest & TB 05 (5.95%), Paediatrics 03(3.5%) and Obs & Gynae 01 (1.2%), respectively[[Figure 5]].
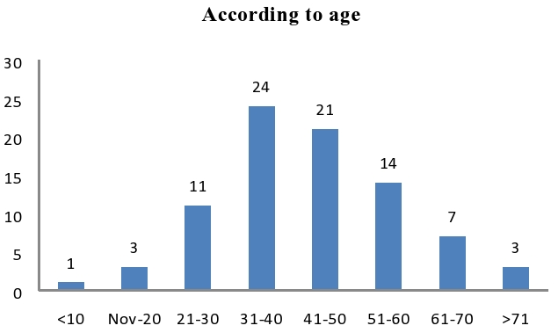
Maximum samples received from middle age group (30-50)[[Figure 6]].
All ESBL positive Pseudomonas aeruginosa isolates showed high resistance to ciprofloxacin 79(94.05%), Gentamicin 61(72.62%) and tobramycin 60 (71.43%). Resistance was low to combination drugs like cefoparazone +salbactum 17(20.24%) and piperacillin + Tazobactum 14 (16.67%). These strains also showed resistance to carbapenems like Imipenem 15 (17.86%), which were found to be the precious weapon against Pseudomonas aeruginosa infections and this, is an alarming sign. All the isolates showed 100% sensitive to Polymyxin B [[Table 1]]. All isolates from urine samples showed (100%) resistance to Norfloxacin.
|
S. No. |
Drug |
No. of Resistance sample |
Percentage |
|
1 |
Ciprofloxacin (5 μg) |
79 |
94.05 |
|
2 |
Gentamicin (10 μg) |
61 |
72.62 |
|
3 |
Tobramycin (10 μg) |
60 |
71.43 |
|
4 |
Cefaperazone-Salbactum (75/10 μg) |
17 |
20.24 |
|
5 |
Piperacillin-Tazobactam (100/10 μg) |
14 |
16.67 |
|
6 |
Imipenem (10 μg) |
15 |
17.86 |
|
7 |
Norfloxacin (10 μg) |
00 |
0.00 |
|
8 |
Polymyxin B (300U) |
00 |
0.00 |
Discussion
Our study showed 84 (26.08%) isolates were ESBL producer which is very similar to other studies, by Prashant et al.,[8] and Agarwal et al.,[9] which were 22.22% & 20.27% respectively Whereas, high percentage of isolates were ESBL producer (45.19%) by Senthamarai S. et al.,[10] 42.30% ESBL producer were observed in the study of Varun Goel et al.,[11]
In the present study, In Patient Department (IPD) 77(91.67%) and only 07(8.33%) samples were of OPD patients. A similar observation was made by Shampa Anupurba et al.,[12] and Prashant et al.[8] They expressed their view that the duration of the hospital stay was directly proportional to a higher prevalence of the infection, since the rate of isolation of the organisms was higher in indoor patients than in outdoor patients.
Isolates found to be more distributed among age groups between 31 to 50 years of age 41 (27.63%). Similar high prevalence in middle age group is reported by Senthamarai S et al.,[10]
Pus (35.71%) was the main source of P. aeruginosa followed by Endotraceal aspirate (25%), urine (13.10%), sputum (10.71%), drain tip (9.52%),blood (4.76%) & cerebrospinal fluid (1.19%). Similar results had been reported by Manzoor et al.,[13] and Senthamarai S. et al.,[10] Although Amandeep Kaur et al.,[14] reported more prevalence in urine samples than respiratory samples.
ESBL-producing bacteria are frequently resistant to many other classes of antibiotics, including aminoglycosides and fluoroquinolones. This is due to the coexistence of genes encoding drug resistance to other antibiotics on the plasmids which encode ESBL.[15] This fact has also been observed in our study.
Resistance was low to combination drugs like cefoparazone +salbactum (20.24%) and piperacillin + Tazobactum (16.67%) as compare to Suprakash Das et al.,[16] and Senthamarai S.[10]
In our study susceptibility to Imipenem is in accordance with Manzoor et al.,[14] however, it is lower compared to the 100% susceptibility found in ESBL-producing gram-negative isolates By Ullah et al.,[17] decreased susceptibility to Imipenem is a matter of great concern and indicates the urgent need for improved infection control strategies.
All the isolates showed 100% sensitive to Polymyxin B similar to study done by Kalaivani R et al.,[18]
Conclusion
Phenotypic combined Disc Diffusion test is widely used due to its simplicity and ease to perform and interpret this test. The present study was done to know the prevalence of ESBL mediated resistance in P. aeruginosa that causes a therapeutic challenge in health care. Most of these isolates were from hospitalized patients which indicate more chances of their nosocomial predominance. The presence of ESBL positivity in out patients is alarming sign for community spread and increase in community-acquired ESBLs.
To reduce the problem of emerging ESBL positive P. aeruginosa, we need team effort from the microbiologists, clinicians and infection control team.
Sources of Funding
None.
Conflict of Interest
None.
References
- Strauß LM, Dahms C, Becker K, Kramer A, Kaase M, Mellmann A. Development and evaluation of a novel universal β-lactamase gene subtyping assay for blaSHV, blaTEM and blaCTX-M using clinical and livestock-associated Escherichia coli. J Antimicrob Chemother. 2015;70(3):710-5. [Google Scholar]
- Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39(6):1211-33. [Google Scholar]
- Ambler RP. . The Structure of β-Lactamases. Philosophical Transactions of the Royal Society of London B. 1036;289:321-352. [Google Scholar]
- Prashanth K, Singh SK, Kanungo R, Sharma S, Shashikala P, Joshi S. Correlations between genotyping and antibiograms of clinical isolates ofPseudomonas aeruginosafrom three different south Indian hospitals. Indian J Med Microbiol. 2010;28(2):130-7. [Google Scholar]
- E K, S A, W J, P S, W W. . Colour Atlas and textbook of Diagnostic Microbology. 2006. [Google Scholar]
- . Clinical Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility testing. . . [Google Scholar]
- Wayne PA. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. Clin Lab Stand Inst. 2015;35(3):M100-S25. [Google Scholar]
- Peshattiwar PD, Peerapur BV. ESBL and MBL Mediated Resistance in Pseudomonas Aeruginosa. J Clin Diag Res. 2011;5(8):1552-4. [Google Scholar]
- Aggarwal R, Chaudhary U, Bala K. Detection of extended-spectrum β-lactamase in Pseudomonas aeruginosa. Indian J Pathol Microbiol. 2008;51(2):222-4. [Google Scholar]
- Senthamarai S, Reddy SKA, Sivasankari S, Anitha C, Somasunder V, Kumudhavathi M. Resistance Pattern of Pseudomonas aeruginosa in a Tertiary Care Hospital of Kanchipuram. J Clin Diagn Res. 2014;8(5):DC30-2. [Google Scholar]
- Goel V, AS, HS, Desai K. Prevalence of extended-spectrum beta-lactamases, AmpC beta-lactamase, and metallo-beta-lactamase producing Pseudomonas aeruginosa and Acinetobacter baumannii in an intensive care unit in a tertiary care hospital. J Sci Soc. 2013;40(1):28-31. [Google Scholar]
- Anupurba S, Battacharjee A, Garg A, Ranjansen M. The antimicrobial susceptibility of Psuedomonas aeruginosa isolated from wound infections. Indian J Dermatol. 2006;51(4). [Google Scholar]
- Ahmad M, Hassan M, Khalid A, Tariq I, Asad MHHB, AS. Prevalence of Extended Spectrum 𝛽-Lactamase and Antimicrobial Susceptibility Pattern of Clinical Isolates of Pseudomonas from Patients of Khyber Pakhtunkhwa, Pakistan. Biomed Res Int. 2016;2016. [Google Scholar]
- Kaur A, Singh S. Prevalence of Extended Spectrum Betalactamase (ESBL) and Metallobetalactamase (MBL) Producing Pseudomonas aeruginosa and Acinetobacter baumannii Isolated from Various Clinical Samples Hindawi. J Pathog. 2018. [Google Scholar]
- Nathisuwan S, Burgess DS, Lewis JS. Extended-Spectrum β-Lactamases: Epidemiology, Detection, and Treatment. Pharmacotherapy. 2001;21(8):920-8. [Google Scholar]
- Das S, Azad M, Bimal K, Oraon V. Antimicrobial Susceptibility Pattern of Pseudomonas aeruginosa with Special Reference to ESBL Producers from Various Clinical Samples at a Tertiary Care Center in Bihar. Int J Res Rev. 2020;7(1):2454-237. [Google Scholar]
- Ullah F, Malik SA, Ahmed J. Antimicrobial susceptibility and ESBL prevalence in Pseudomonas aeruginosa isolated from burn patients in the North West of Pakistan. Burns. 2009;35(7):1020-5. [Google Scholar]
- Kalaivani R, Shashikala P, Devi S, Prashanth K, Saranathan R. Phenotypic assays for detection of esbl and mbl producers among the clinical isolates of multidrug resistant pseudomonas aeruginosa from a tertiary care hospital. Int J Cur Res Rev. 2013. [Google Scholar]
How to Cite This Article
Vancouver
Tewari S, Kumar A, Rani E. Prevalence of extended-spectrum β-lactamase in Pseudomonas aeruginosa in tertiary care centre [Internet]. Indian J Microbiol Res. 2020 [cited 2025 Oct 11];7(3):226-229. Available from: https://doi.org/10.18231/j.ijmr.2020.041
APA
Tewari, S., Kumar, A., Rani, E. (2020). Prevalence of extended-spectrum β-lactamase in Pseudomonas aeruginosa in tertiary care centre. Indian J Microbiol Res, 7(3), 226-229. https://doi.org/10.18231/j.ijmr.2020.041
MLA
Tewari, Swati, Kumar, Alok, Rani, Ekta. "Prevalence of extended-spectrum β-lactamase in Pseudomonas aeruginosa in tertiary care centre." Indian J Microbiol Res, vol. 7, no. 3, 2020, pp. 226-229. https://doi.org/10.18231/j.ijmr.2020.041
Chicago
Tewari, S., Kumar, A., Rani, E.. "Prevalence of extended-spectrum β-lactamase in Pseudomonas aeruginosa in tertiary care centre." Indian J Microbiol Res 7, no. 3 (2020): 226-229. https://doi.org/10.18231/j.ijmr.2020.041
