- Visibility 28 Views
- Downloads 7 Downloads
- DOI 10.18231/j.ijmr.2022.034
-
CrossMark
- Citation
Anti-viral activity of suramin against influenza A virus in A549 cells
Introduction
Influenza A virus (IAV) an enveloped, segmented, negative strand RNA virus belongs to the family of Orthomyxoviridae, a major respiratory pathogen and responsible for causing number of global and local outbreaks.[1] IAV infections still persists challenges clinically especially in high-risk patients such as elderly, immuno-compromised patients and in children. Currently, there are three classes of anti-influenza drugs with different antiviral mechanisms, including M2 ion channel blockers (amantadine and rimantadine), viral RNA synthesis inhibitors (ribavirin and T-705) and neuraminidase (NA) inhibitors (oseltamivir and zanamivir).[2] With evolution of NA inhibitor-resistant influenza viruses have also emerged, thus limiting the future utility of NA inhibitors. Suramin, a symmetric polyanionic naphthylurea and market-authorized drug for trypanosomiasis with wide range of potential applications in viral diseases, cancer and autism.[3] The broad-spectrum antiviral efficacy of suramin has been demonstrated in many viruses like chikungunya and Ebola virus.[4] Due to emergence of oseltamivir (Tamiflu) resistance strain, our aim was to evaluate suramin as novel, potent anti-influenza molecule.
Materials and Methods
Cells, virus, reagents, antibodies and drug
Human lung epithelial cells (A549) were cultured in Dulbeco’s Modified Essential Medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen) at 37˚C and 5% CO2. IAV strain A/Puerto Rico/8/1934(H1N1) was used for the study. Suramin (Sigma) was dissolved in nuclease free water and a stock solution of 100 mg/ml was prepared and stored at -20˚C until use. Oseltamivir was purchased from Sigma. Rabbit anti-PB1 antibody, Trizol reagent, BCA Protein Assay Kit was purchased from Invitrogen. RIPA Buffer (10×) and protease inhibitor cocktail (100×), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and HRP conjugated anti-rabbit secondary IgG antibody were procured from Cell Signalling Technology. MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium), Dimethyl sulfoxide (DMSO) was purchased from Sigma. Bovine serum albumin (BSA) was purchased from Himedia. Viral RNA Mini Kit was procured from Qiagen. Complementary DNA (cDNA) synthesis kit was purchased from Promega. SYBR (2×) green master mix purchased from Affymatrix. Gene specific primers were synthesized from Eurofins.
Cytotoxicity Assay
A549 cells/well (1x104) were seeded in 96 wells tissue culture plate and incubated at 37˚C, 5% CO2 for 24hr. Varying concentrations of suramin (1000 µg/ml to 3.9 µg/ml) was added to the cells and incubated for 24hr at 37˚C. Similarly, serially diluted concentration of oseltamivir (400 µg/ml to 0.195 µg/ml) was added. Post 24hr incubation, MTT reagent was added and incubated for 3hr at 37˚C at dark. DMSO was added to dissolve the formazan crystals and absorbance was recorded at 570nm using Epoch 2 microplate spectrophotometer (Biotek).
Virus infection and treatment
A549 cells were seeded in 6 well tissue culture plate at a density of (3x105) cells/well and infected with IAV at multiplicity of infection 1 (MOI 1) and treated with suramin from1000 µg/ml to 125 µg/ml by co-incubation for 1 hr at 37˚C. Similarly, A549 cells were infected with IAV at MOI 1 and treated with Oseltamivir at concentrations from 200 µg/ml to 25 µg/ml. Following infection, cells were washed with 1× PBS and DMEM containing 1% FBS was added and further incubated for 24 hr at 37˚C.
Quantitative real time RT-PCR
Cells were lysed using Trizol reagent for RNA isolation and viral RNA was isolated using viral RNA Mini Kit according to manufacturer’s instruction (Qiagen). The mRNA was quantified and 500ng was used for cDNA synthesis in a 20µl reaction as per manufacturer’s protocol (Promega). The relative expression of viral gene was detected by CFX96 real time PCR (Biorad) using 2× SYBR green master mix (Affimetrix). The gene of interest was normalized to GAPDH and the data was expressed as fold change using 2ΔΔCt. The following thermal cycling profile was used for the PCR analysis: 95°C for 10 minutes, 95°C for 15 seconds, and 55°C for 15 seconds, 60°C for 1 min 72°C for 30 seconds for 35 cycles.
Western blotting
Cells were lysed using RIPA buffer containing protease inhibitor cocktail as per manufacturer’s instructions (Cell Signalling Technology). Total protein was quantified using BCA reagent and mixed with 6× Laemmli Buffer (375mM Tris-HCl pH6.8, 6% SDS, 50% glycerol, 9% 2-mercaptoethanol, 0.03% bromophenol blue). Equal concentration (50 µg/well) of protein were separated on 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE). After separation, proteins were transferred onto Polyvinylidene fluoride (PVDF) membrane using semi-dry transfer system (Biorad). The membrane was blocked with 5% BSA containing 0.1% tween 20 for 1hr at room temperature (RT) and probed with anti-PB1 (1:2000 dilution) antibody for 3hr at RT. The membrane was washed with 1× PBS and probed again with HRP conjugated secondary anti-rabbit (1:1000 dilution) IgG antibody for 1hr at RT in suramin treated cells. Cells treated with oseltamivir was probed with rabbit anti-haemagglutinin (HA) antibody. GAPDH was used as housekeeping protein. Expression was detected with chemiluminescence detection kit (Biorad) and visualized using chemidoc imaging system (Biorad).
Cytopathic effect (CPE inhibition)
A549 cells were seeded in 12 well tissue culture plate at a density of (1 x 105) cells/well. Infection and suramin treatment were given as described above. Post infection, cells were washed with 1X PBS and re-suspended in 1% serum DMEM for further incubation at 37°C for 24 hr and viewed at 20× magnification and images were captured using Nikon Eclipse bright field microscope.
Statistical analysis
Graph Pad Prism 9 was used for statistical analysis. The result obtained was expressed in mean ± SD. One-way ANOVA was used to analysed the data and p value <0.05 were considered significant. All the experiments were done in triplicates and data is represented as mean ± SD.
Results
Cell viability assay of suramin and oseltamivir
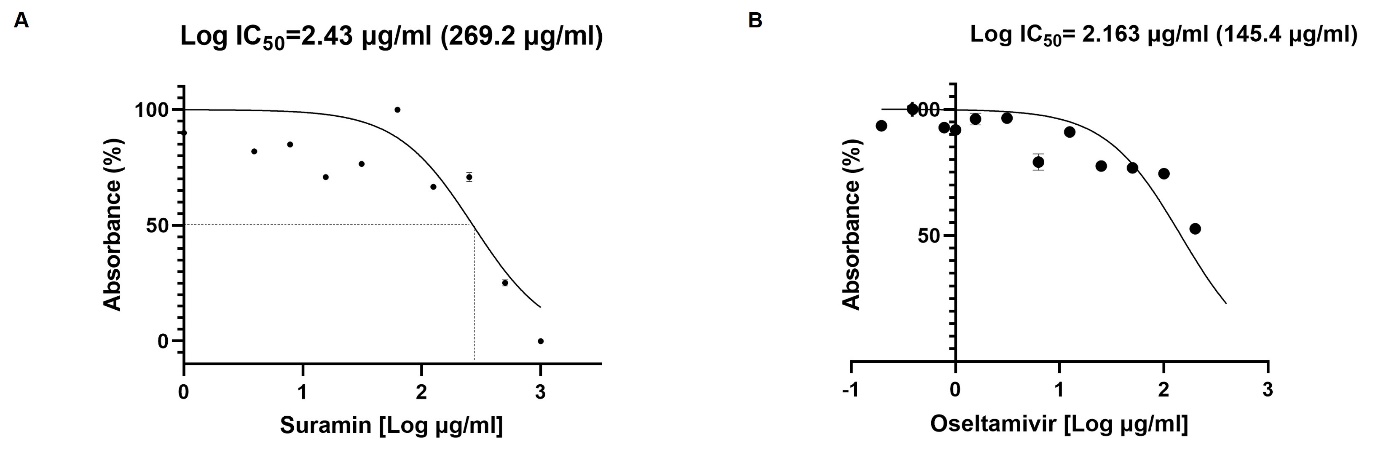
The cytotoxicity of suramin and oseltamivir on A549 cells was determined by the MTT assay. The results showed that suramin had a half-maximal toxic concentration of 269.2 µg/ml (Log IC50=2.43 µg/ml) and IC50 for oseltamivir was 145.4 µg/ml (Log IC50=2.16 µg/ml) in A549 cells at 24hr ([Figure 1] A,B). Thus, for suramin the concentrations of 1000, 500, 250 and 125 µg/ml was used in all experiments. Similarly, for oseltamivir the concentrations of 200, 100, 50 and 25 µg/ml was used to evaluate the antiviral activity.
In vitro anti-viral activity of suramin against A/PR/8
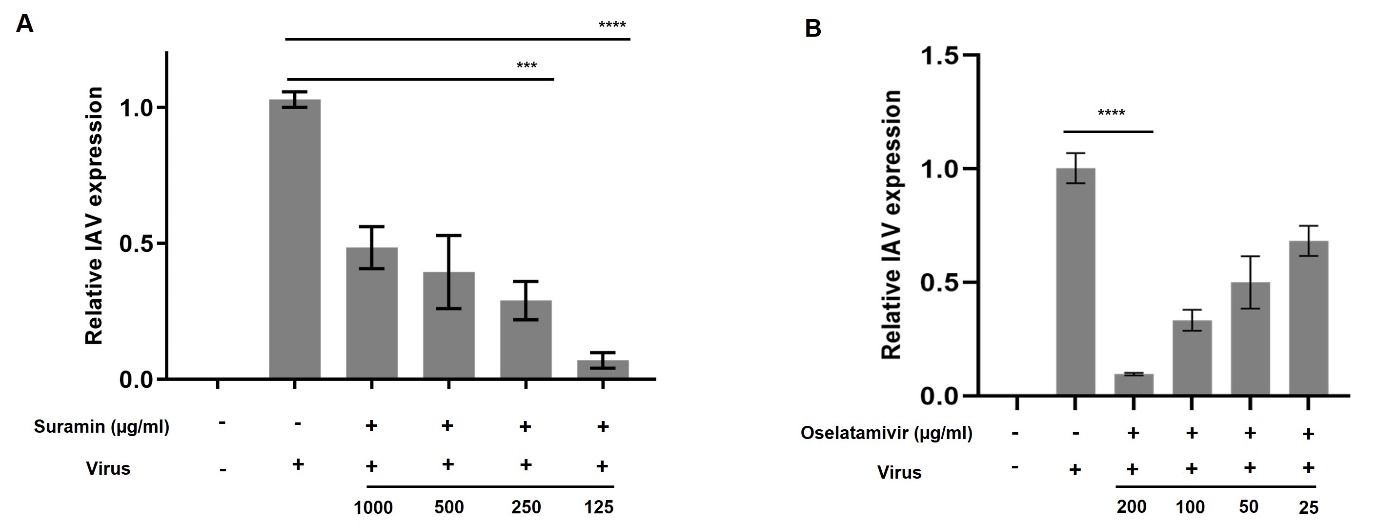
To determine whether suramin has antiviral activity against IAV, we analysed changes in expression of viral RNA transcripts using quantitative real-time PCR (qPCR) at 24hr. Suramin at all the concentrations viral RNA.Hexhibited maximum anti-viral efficacy at 250 and 125 µg/ml (p < 0.0001) ([Figure 2] A) as positive control antiviral drug that showed anti influenza activity against A/PR/8 in A549 cells which showed maximum inhibition at 200 µg/ml (p<0.0001) ([Figure 2]B).
Suramin inhibited replication of A/PR/8
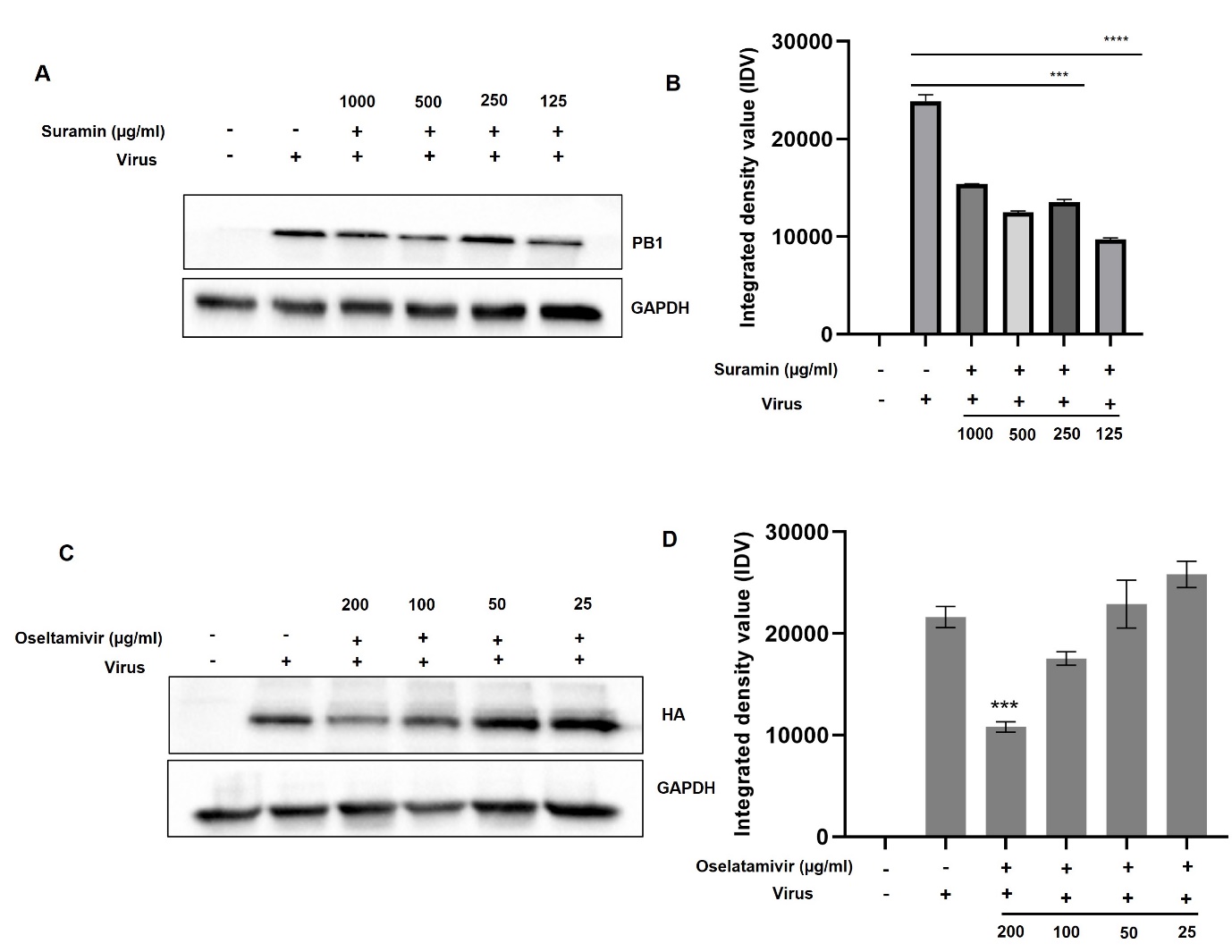
The inhibitory effect of suramin and oseltamivir against IAV was further validated via western blotting using PB1 and HA specific antibody. The expression of PB1 decreased significantly after treatment with suramin at 250 and 125 µg/ml, corroborated with our qPCR result (p < 0.0001) ([Figure 3]A and 3B). Similarly, cells treated oseltamivir also showed inhibition when probed with HA specific antibody (p < 0.001), which indicates suramin exhibited superior anti-viral activity against A/PR/8 H1N1 ([Figure 3]C and 3D).
Suramin inhibits CPE
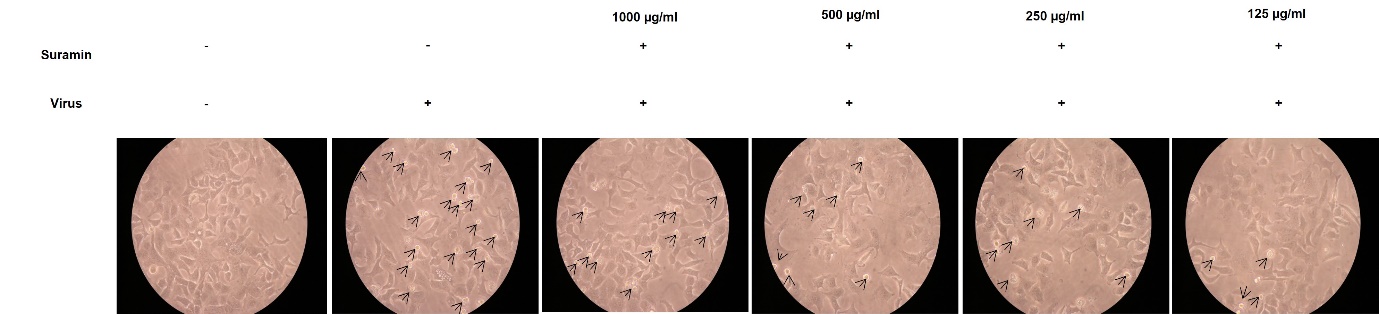
To further confirm the hypothesis, cytopathic effect (CPE) assay was performed at 24hr post-infection. Bright field microscopic images demonstrated that in control, A549 cells showed no development of CPE. Cells infected with IAV only showed rapid development of CPE. Treatment with suramin reduced CPE formation and at 250 and 125 µg/ml significantly ([Figure 4]). Thus, confirming the anti-viral efficacy of suramin against IAV.
Discussion
Oseltamivir is the most used anti-influenza drug worldwide. However, due to emergence of Oseltamivir-resistant viral strains, which harbour mutations, such as the H274Y amino acid substitution.[5] Thus, it is necessary to establish a low toxic and potent antiviral to overcome the resistance of influenza virus. Suramin have demonstrated antiviral properties by inhibiting helicase activity of NS3 of dengue virus.[6] Suramin neutralizes Enterovirus 71 (EV71) virus particles prior to cell attachment.[7] Decreased viral load was observed in SARS-CoV-2 replication when treated with suramin possibly by inhibiting binding or entry of the virus.[8] In this study, suramin significantly reduced the expression of PB1 protein which is a part of RNA dependent RNA polymerase (RdRp) and also an important factor in pathogenesis of influenza infection. Suramin exhibited potential efficacy against ZIKA virus infection at concentration of 50µg/ml at 24 hr post-infection. The mechanism of action demonstrated that, suramin was unable to inactivate ZIKA virus particles, but hinder with virus adsorption, entry and post-infection events.[9] Dose dependent inhibitory activity of suramin in Chikungunya virus shows reduction in viral RNA, capsid protein expression and RNA expression. At concentration of 175 µM and 350 µM demonstrated significant reduction in RNA and capsid protein expression analysis and also found entry blocker. The inhibitory effects of suramin on CHIKV virus surface glycoproteins showed viral entry and transmission was prohibited by binding with CHIKV E1/E2 glycoproteins.[10] Suramin inhibited human parainfluenza type-3 virus (hPIV-3) in epithelial cells at lower concentration.[11] Similar to our RT-PCR and western blot results, CPE induced by IAV in suramin treated cells (0.25mg/ml and 0.125mg/ml) was lesser. Overall, our results suggest strong antiviral activity of suramin at viral RNA and protein level at 250 µg/ml and 125 µg/ml compared to Oseltamivir against IAV and could be a broad spectrum anti-viral drug.
Conclusion
Suramin, a symmetric polyanionic naphthylurea used to treat African sleeping sickness, have some anti-viral efficacy against various viruses. Our study demonstrated that, suramin exhibited anti-viral activity by reducing virus infectivity at lower concentration thus, suramin might be acceptable for the treatment of influenza virus infections compare to oseltamivir as long as there is no better treatment available. However, appropriate preclinical studies will further strengthen the data. To the best of our knowledge, this is the first data which demonstrated significant anti-viral activity of suramin against influenza A virus. Therefore, we believe this investigation may be useful in initiating further research to develop suramin as novel therapeutic intervention for treatment of IAV.
Data Availability Statement and Citations
Data for the study is included in the article.
Source of Funding
None.
Conflicts of Interest
The authors declare no competing interests.
Author’s Contribution
TN (Tanusri Nandi) and MK (Madhu Khanna) conceived the idea and designed the study. TN performed all the experiments and wrote the manuscript. MK edited the final version of the manuscript. All the authors have read and approved the final manuscript.
Acknowledgements
The authors wish to thank Vallabhbhai Patel Chest Institute, University of Delhi for providing laboratory facilities.
References
- F Krammer, GJD Smith, RAM Fouchier, M Peiris, K Kedzierska, PC Doherty. Influenza. Nat Rev Dis Primers 2018. [Google Scholar]
- L Amarelle, E Lecuona, JI Sznajder. Anti-Influenza Treatment: Drugs Currently Used and Under Development. Arch Bronconeumol 2016. [Google Scholar]
- N Wiedemar, DA Hauser, P Mäser. 100 Years of Suramin. Antimicrob Agents Chemother 2020. [Google Scholar]
- L Henß, S Beck, T Weidner, N Biedenkopf, K Sliva, C Weber. Suramin is a potent inhibitor of Chikungunya and Ebola virus cell entry. Virol J 2016. [Google Scholar]
- SR Kim, MS Jeong, SH Mun, J Cho, MD Seo, H Kim. Antiviral Activity of Chrysin against Influenza Virus Replication via Inhibition of Autophagy. Viruses 2021. [Google Scholar]
- C Basavannacharya, SG Vasudevan. Suramin inhibits helicase activity of NS3 protein of dengue virus in a fluorescence-based high throughput assay format. Biochem Biophys Res Commun 2014. [Google Scholar]
- P Ren, G Zou, B Bailly, S Xu, M Zeng, X Chen. The approved pediatric drug suramin identified as a clinical candidate for the treatment of EV71 infection-suramin inhibits EV71 infection in vitro and in vivo. Emerg Microbes Infect 2014. [Google Scholar]
- C Salgado-Benvindo, M Thaler, A Tas, NS Ogando, PJ Bredenbeek, DK Ninaber. Suramin Inhibits SARS-CoV-2 Infection in Cell Culture by Interfering with Early Steps of the Replication Cycle. Antimicrob Agents Chemother 2020. [Google Scholar]
- CW Tan, IC Sam, WL Chong, VS Lee, YF Chan. Polysulfonate suramin inhibits Zika virus infection. Antiviral Res 2017. [Google Scholar]
- YJ Ho, YM Wang, JW Lu, TY Wu, LI Lin, SC Kuo. Suramin Inhibits Chikungunya Virus Entry and Transmission. PLoS One 2015. [Google Scholar]
- H Yan, H Wang, L Ma, X Ma, J Yin, S Wu. Cirsimaritin inhibits influenza A virus replication by down regulating the NF-κB signal transduction pathway. Virol J 2018. [Google Scholar]
- Introduction
- Materials and Methods
- Cells, virus, reagents, antibodies and drug
- Cytotoxicity Assay
- Virus infection and treatment
- Quantitative real time RT-PCR
- Western blotting
- Cytopathic effect (CPE inhibition)
- Statistical analysis
- Results
- Cell viability assay of suramin and oseltamivir
- In vitro anti-viral activity of suramin against A/PR/8
- Suramin inhibited replication of A/PR/8
- Suramin inhibits CPE
- Discussion
- Conclusion
- Data Availability Statement and Citations
- Source of Funding
- Conflicts of Interest
- Author’s Contribution
- Acknowledgements
