- Visibility 67 Views
- Downloads 8 Downloads
- DOI 10.18231/j.ijmr.2022.039
-
CrossMark
- Citation
Phytochemical screening and antibacterial activity of Trachyspermum ammi L. against certain bacterial species
- Author Details:
-
Aparajita Gupta *
-
Mannu Kumari
-
Shabnam Sharma
Introduction
For thousands of years, nature has provided a source of medicinal plants, from which a vast variety of life-saving pharmaceuticals have been developed. India has a wide variety of plants. Medicinal plants are known to have therapeutic properties and are used to treat a variety of human illnesses. The word "herbal drug" refers to a component or parts of a plant (leaves, flowers, seeds, roots, barks, stems, and so on) that are widely used to make medications. Plants were first used in India's ancient medicinal system in the Rig Veda, which was written between 4500 and 1600 B.C. The Ayurveda, which was written between 2800 and 600 B.C., discussed the medicinal properties of a various drug plants.[1] Natural medications are the primary source of healthcare for nearly 80% of the world's population, according to a World Health Organization report.
The "antibiotic age" began with the discovery of penicillin. Antibiotics have the potential to treat and cure microbial illness, which has resulted in a significant reduction in the number of deaths, a significant improvement in the quality of life for many patients, a decrease in childhood mortality, an increase in life expectancy, and the saving of many lives.[2] New antibiotics were developed after the discovery of penicillin. Some of the most widely used antibiotics for treatment are cephalosporins, aminoglycosides, glycopeptide antibiotics and quinolones.
The growth of multidrug resistance (MDR) bacteria strains in recent years has occurred from the development of resistance in bacteria to various classes of antibiotics.[3] Methicillin-resistant S. aureus (MRSA), penicillin-resistant Streptococcus pneumoniae (PRSP), vancomycin-resistant Enterococci (VRE), Salmonella, Shigella, and Klebsiella, as well as other multidrug-resistant Pseudomonas,Campylobacter, and Mycobacterium tuberculosis, are examples of MDR bacteria.[4], [5], [6], [7]
A surge in multi-drug resistance microbes has prompted increased interest in the search for new pharmaceutical formulations from natural sources, including plants. As a result, alternative plant-based antimicrobial drugs are needed to treat infectious diseases.[8] Medicinal plants hold a lot of potential in the fight against multidrug-resistant microorganisms. [9] Tannins, alkaloids, flavonoids, saponins, and glycosides are physiologically active secondary metabolites and have broad spectrum antibacterial properties.[10] They offer a lot of therapeutic potential and could be the basis for novel drug development.[11], [12] Therefore there is need to evaluate the crude extracts of medicinal plants for their antimicrobial properties.
Trachyspermum ammi L. or T. ammi belongs to (Apiaceae family) is a traditional medicinal plant with therapeutic properties. T. ammi seeds are used to treat a variety of gastrointestinal ailments in traditional Indian medicine system. A hot and dry fomentation of the seeds is applied to the chest to treat asthma, and a paste of powdered seeds is used externally to relieve colic pains.[13] Aqueous extract of the seeds of the ajwain plant is a common therapy for diarrhoea. T.ammi seeds are employed as stomachic, carminative, expectorant, antiseptic, anti-amoebiasis, and antibacterial agents in medicine. Seeds have also been linked to the prevention of stomach cancers, aches, and piles.[14] There are a variety of ajwain ayurvedic formulations available to treat worm infections.[15]
Materials and Methods
Collection of plant material and preparation of plant extracts
T. ammi seeds were obtained from a local market in Chunni Kalan, Fatehgharh Sahib. A mixer grinder was used to ground the ajwain seeds into a coarse powder. Seeds powder was ground and stored in sterile polythene bags at room temperature. T. ammi seeds were extracted using several solvents (methanol, ethanol, and acetone). 40 grams of ground plant material were extracted for 24-48 hours in a Soxhlet Extractor with 300 ml of extraction solvent. Finally, the extracted extract was filtered through sterile Whatman No. 1 filter paper, and the solution was evaporated to dryness under controlled temperature conditions to yield a final volume of 40 ml. The final extract concentration was made at level in which one gram of powdered plant material was equal to 1ml of extract solution. [16] The resulted extract solution was labelled as a 100% concentrated drug solution. This 100% drug solution was further diluted with sterile distilled water to obtain 75%, 50% and 25% concentrations.
Bacterial cultures
The bacterial culture used in the present investigation includes Staphylococcus aureus (MTCC code 3160), Pseudomonasaeruginosa, (MTCC code 3542), Escherichia coli, (MTCC code 443) Klebsiella pneumonia, (MTCC code 9544) and Bacillus cereus (MTCC code 430). All the culture were collected from Microbial Type Culture Collection (MTCC), IMTECH Chandigarh, India.
Method
Test for antibacterial activity
The antibacterial activity of plant extracts was tested using the agar well diffusion method.[17] Inoculums (nutrient broth culture of test organism) was added in nutrient agar medium.2 ml bacterial suspension was added to 100 ml of nutrient agar medium. The flask was gently rotated to ensure that the test organisms were distributed evenly. The inoculated culture media was then poured into sterile petri plates and allowed to solidify completely in a laminar air flow. Sterile cork borer of 6mm in diameter was used to make 5 wells in the set of each petridishes with 4 well in the periphery and one well in the center. 0.1ml (100µm) solution from each differ concentration i.e. (100%, 75%, 50% and 25%) of plant extract was added to four peripheral wells. Central wells were filled with 0.1ml solution of control. Methanol, ethanol and acetone were used as control for methanolic, ethanolic and acetonic extract. These petridishes were then incubated at 37ºC for 24 hours. After incubation, the diameter of zones of inhibition were measured and tabulated for each test bacterial strain. Each sample was assayed in triplicate and value was measured and recorded. Inhibition zone was measured in millimeters with the ruler. It was measured from center of the well to the edge of the area with no growth (zero growth) and was multiplied by two. Further, average value of inhibition zones was calculated. Effective inhibition zone was calculated by deducting the well size (corkborer size) from average value of inhibition zone.
Phytochemical analysis
Tests for flavonoids
Alkaline Reagent Test: 2ml of 2.0 percent NaOH was added to the crude extract. The presence of flavonoids was revealed by the appearance of a yellow tint that faded to colorlessness when 2 drops of diluted acid were added.[18]
Test for tannins
Ferric chloride test: To 2 mL of extract, a few drops of 10% ferric chloride solution were added. The presence of gallic tannins was indicated by the emergence of a blackish blue colour, whereas the presence of catechol tannins was indicated by the appearance of a green-blackish colour.[19]
Test for alkaloids
Mayer’s test: To 1 mL of extract, add 1 mL of Mayer's reagent. The presence of alkaloids is indicated by the presence of a white yellow or cream coloured precipitate.[20]
Test for saponins
Foam test: 1 mL extract solution was diluted to 20 mL with distilled water and agitated for 15 minutes in a graduated cylinder. The presence of saponins is indicated by the appearance of foam. [21]
Test for glycosides
Keller-Kiliani test: The crude extract was mixed with 2 mL of glacial acetic acid containing 1-2 drops of 2% FeCl3solution. After that, the extract was poured into a second test tube containing 2ml of concentrated H2SO4. The presence of cardiac glycosides was shown by the formation of a brown ring at the interface.[22]
Result and Discussion
In the present investigation, three different solvents i.e. methanol, ethanol and acetone were used to extract different phytoconstituents from T. ammi. Antibacterial activity was carried against all bacterial test strains i.e. S. aureus, E. coli, B. cereus, P. aeruginosa, K. pneumoniae in four different concentrations i.e. 100%, 75%, 50% and 25%. 100% concentrations means the extract without any dilutions. Solvent was used as control for each extract. The antibacterial activity in term of effective inhibition zone was recorded after deducting well size (6mm) from average value of inhibition zones.
Antibacterial activity of methanol extract of seeds of Trachyspermum ammi.
The maximum antibacterial activity at 100% concentration was recorded against Pseudomonas aeruginosa (20.3mm) Staphylococcus aureus (20.3mm), E.coli (19.3mm), Bacillus cereus(19mm), Klebsiella pneumoniae (17mm) followed by. At 75% maximum inhibitory effect was found against Staphylococcus aureus (18.6mm), P.aeruginosa (17mm), E. coli(16.3mm), Bacillus cereus (15mm) and minimum against K.pneumoniae (13.6mm). whereas at 50% concentration maximum inhibition was found against S.aureus (15mm), P.aeruginosa (14.3mm), E.coli (14mm), B.cereus (12.3mm) and minimum against K.pneumoniae(12mm). At 25% concentration maximum inhibition was found against S.aureus (12.6mm), E.coli (12.3mm), P.aeruginosa (11mm), K. pneumoniae (10.3mm) and minimum against B.cereus (9mm). Results have been presented in [Table 1], [Figure 1].
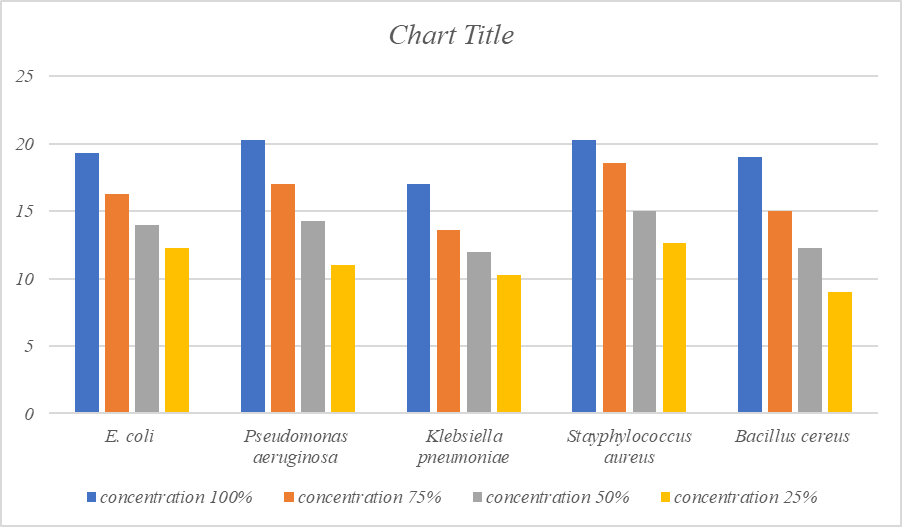
Antibacterial activity of ethanol extract of Trachyspermum ammi
The maximum antibacterial activity at 100% concentration was recorded against Pseudomonas aeruginosa (22.6mm), followed by Bacillus cereus (22.6mm), Klebsiella pneumoniae (21.3mm) Staphylococcusaureus(18mm) and minimum against E.coli (12mm) followed by. At 75% maximum inhibitory effect was found against P.aeruginosa (20mm), K.pneumoniae (17.3mm), Bacillus cereus (17mm), S.aureus (15mm) and minimum against E.coli (10mm) whereas at 50% concentration maximum inhibition was found against P.aeruginosa (15.6mm), B.cereus (14mm), K.pneumoniae (13mm), S.aureus (12mm) and minimum against E.coli (7mm). At 25% concentration maximum inhibition was found against S.aureus (9mm), K.pneumoniae(9mm), P.aeruginosa (8.3mm), Bacillus cereus (8mm) and minimum against E.coli (4.3mm). Results have been presented in [Table 1], [Figure 2], and photoplate-1.
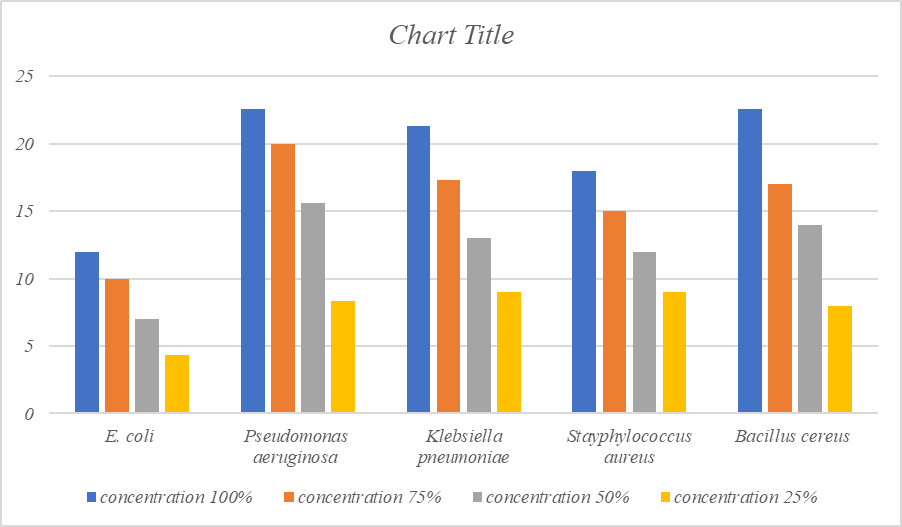
Antibacterial activity of acetone extract of Trachyspermum ammi
The maximum antibacterial activity at 100% concentration was recorded against Staphylococcus aureus (20.3mm), Klebsiella pneumoniae (13.3mm), Pseudomonas aeruginosa (13.3mm), Bacillus cereus (11.6mm), and minimum against E.coli (11.3mm) followed by. At 75% maximum inhibitory effect was found against S.aureus (17mm), K.pneumoniae (10.6mm), P. aeruginosa (10.6mm), Bacillus cereus (10mm), and minimum against E.coli (9.3mm). Whereas at 50% concentration maximum inhibition was found against S.aureus (11mm) P. aeruginosa (9mm), K. pneumoniae (8.3mm), B.cereus (7.6mm) and minimum against E.coli (7.6mm). At 25% concentration maximum inhibition was found against S.aureus (6mm), E.coli (5mm), B. cereus (4.6mm), K. pneumoniae (4.3mm) and minimum against P. aeruginosa (3.6mm). Results have been presented in [Table 1], [Figure 3].
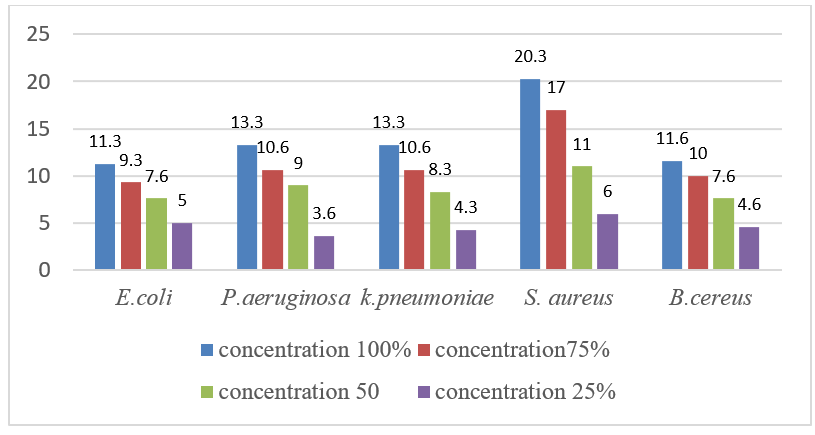
|
Extract Type |
Test Organisms |
Effective Zone of Inhibition (mm) |
|||
|
Extract conc. 100% |
Extract conc. 75% |
Extract conc. 50% |
Extract Conc. 25% |
||
|
Methanol |
E. coli |
19.3 mm |
16.3 mm |
14 mm |
12.3 mm |
|
P. aeruginosa |
20.3 mm |
17 mm |
14.3 mm |
11 mm |
|
|
K. pneumoniae |
17 mm |
13.6 mm |
12 mm |
10.3 mm |
|
|
S. aureus |
20.3 mm |
18.6 mm |
15 mm |
12.6 mm |
|
|
B. cereus |
19 mm |
15 mm |
12.3 mm |
9 mm |
|
|
Ethanol |
E. coli |
12 mm |
10 mm |
7 mm |
4.3 mm |
|
P. aeruginosa |
22.6 mm |
20 mm |
15.6 mm |
8.3 mm |
|
|
K. pneumoniae |
21.3 mm |
17.3 mm |
13 mm |
9 mm |
|
|
S. aureus |
18 mm |
15 mm |
12 mm |
9 mm |
|
|
B. cereus |
22.6 mm |
17 mm |
14 mm |
8 mm |
|
|
Acetone |
E. coli |
11.3 mm |
9.3 mm |
7.6 mm |
5 mm |
|
P. aeruginosa |
13.3 mm |
10.6 mm |
9 mm |
3.6 mm |
|
|
K. pneumoniae |
13.3 mm |
10.6 mm |
8.3 mm |
4.3 mm |
|
|
S. aureus |
20.3 mm |
17 mm |
11 mm |
6 mm |
|
|
B. cereus |
11.6 mm |
10 mm |
7.6 mm |
4.6 mm |
|
|
Control |
-- |
|
|
|
|

Phytochemical analysis of Trachyspermum ammi extract
The extract of seeds was subjected to phytochemical screening for identification of various plant constituents. The phytochemical properties of the plant make it naturally inhibitor to the growth of various microorganisms. Alkaloids were found in methanolic and acetone extract of Trachyspermum ammi seeds. Methanol, ethanol and acetone extracts of seeds were found rich in Tannins. Flavonoids were found on methanol, acetone and ethanol extract of seeds. Saponins was found in ethanol but not detected in methanol and acetone extract seeds. Whereas glycosides was found in acetone but not detected in methanol and ethanol extracts of Trachyspermum ammi.
|
Chemical Constituents |
Test performed |
Methanol extract |
Acetone extract |
Ethanol extract |
|
Alkaloids |
Mayer’s test |
+ |
+ |
- |
|
Tannins |
Ferric chloride test |
+ |
+ |
+ |
|
Flavonoids |
Sulphuric acid test |
+ |
+ |
+ |
|
Saponins |
Foam test |
- |
- |
+ |
|
Glycosides |
Keller’s Killiani test |
- |
+ |
- |
Discussion
Medicinal plants are a rich source of phytochemicals, which are therefore can be used in the treatment of a variety of illnesses. Many biological properties of T. ammi are reported which are due to presence of various phytoconstituents. The organic solvent (methanol, ethanol and acetone) extracts of T. ammi displayed broad spectrum antibacterial activity. The zone of grow inhibition bacteria correspondent to drug concentrations of plant material. A decline trend of inhibition zone was found with the dilution of extract. Maximum antimicrobial efficacy was recorded in ethanolic extract followed by methanolic and acetonic extract. In ethanolic extract maximum efficacy was recorded against P. aeruginosa, B.cereus and minimum against E. coli. Acetonic extract had the highest activity against S. aureus and the lowest against E. coli. Methanolic extract displayed highest inhibition against S. aureus and P. aeruginosa. Similarly Masihet al. (2012) investigated the antibacterial activity of T. ammi seeds ethanolic and acetonic extracts. They observed that Pseudomonas sp., E. coli, Bacillus subtilis, and Staphylococcus aureus were found to be more susceptible to ethanolic extract. While Pseudomonas sp., E. coli, and Bacillus subtilis were all inhibited by an acetonic extract of T. ammi.[23]
Shahidi (2004) reported the antibacterial activity of methanolic extract of T. ammi seeds against P.aeruginosa, Bacillus pumilus, Staphylococcus aureus, S. epidermidis, E. coli, Klebsiella pneumoniae, and Bordetellabronchiseptica.[24] Furthermore, the acetonic extract displayed antibacterialefficacyagainst Enterococcus faecalis, E. coli, K. pneumoniae, P. aeruginosa, Salmonella typhi, S. Typhimurium, Shigellaflexneri, and S. aureus. [25] As a result of this research, the traditional usage of T. ammi seeds to treat a variety of gastrointestinal diseases may be scientifically validated. Qualitative phytochemical examination revealed the presence of alkaloids, flavonoids, saponins, tannins, and glycosides, as shown in [Table 2]. Phytoconstituents are compounds that can have a variety of physicochemical and pharmacological effects. Therefore, their presence confirms the plant’s long history of usage in the treatment of infectious disorders.
Conclusion
Trachyspermum ammi has long been regarded as a valuable medicinal plant. It's been utilized for stomach problems in the past, and it's also used as a flavoring agent. It has a wide spectrum of phytoconstituents that are responsible for a variety of biological properties. Hence the antibacterial activity of T. ammi seeds provides a scientific foundation for their usage as a traditional remedy. T. ammi is a substantial natural antibacterial agent, according to our findings, and can be suggested for the treatment of a variety of microbiological illnesses.
Source of Funding
None.
Conflict of Interest
The authors have no conflicts of interest regarding this investigation.
Acknowledgement
The authors are thankful to Principal and management committe of college or providing the facilities for experimentation.
References
- S Agarwal, S Goyal. In Vitro Antimicrobial Studies of Trachyspermum ammi. Int J Pharma Bio Sci 2012. [Google Scholar]
- LI Bingyun, TJ Webster. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J Orthop Res 2018. [Google Scholar]
- AL Alanis. Resistance to Antibiotics: Are we in the post- Antibiotic Era? . Arch Med Res 2005. [Google Scholar]
- I Ahmad, JNS Yadav, S Ahmad. High level transferable resistance among E. coli. Indian J Ani Sci 1994. [Google Scholar]
- J Davies. Inactivation of antibiotics and the dissemination of resistance genes. Science 1994. [Google Scholar]
- DM Livemore. Beta-Lactamases in Laboratory and clinical resistance. Clin Microbiol Rev 1995. [Google Scholar]
- F Aqil, MSA Khan, M Owais, I Ahmad. Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant S. aureus. J Basic Microbiol 2005. [Google Scholar]
- P Agrawal, V Rai, RB Singh. Randomized, placebo- controlled, single- blind trial of holy basil leaves in patients with noninsulin-dependent diabetes mellitus. Int J Clin Pharmacol Ther 1996. [Google Scholar]
- HJD Dorman, SG Deans. Antimicrobial agents from plants. J Appl Microbiol 2000. [Google Scholar]
- FA Al-Bayati, HF Al-Mola. Antibacterial and antifungal activities of different parts of Tribulusterrestris L. growing in Iraq. J Zhejiang Univ Sci B 2008. [Google Scholar]
- MM Cowan. Plant product as anti-microbial agents. Clin Microbiol Rev 1999. [Google Scholar]
- GE Trease, WC Evan. . Trease and Evans Pharmacognosy 1972. [Google Scholar]
- VK Singh, JN Govil, C Arunachalam. . Recent Progress in Medicinal Plants 2007. [Google Scholar]
- V Krishnamoorthy, MB Madalageri. Bishop weeds (Trachyspermum ammi): an essential crop for north Karnataka. J Med Aromat Plants Sci 1999. [Google Scholar]
- B Ranjan, S Manmohan, SR Singh, RB Singh. Medicinal Uses of Trachyspermum ammi: A Review. Pharma Res 2011. [Google Scholar]
- M Barreto, AT Critchley, CJ Straker. Extract from sea weeds can promote fungal growth. J Basic Microbiol 2002. [Google Scholar]
- SC Bell, WE Grundy. Preparation of agar wells for antibiotic assay. Appl Microbiol 1968. [Google Scholar]
- R Gul, SU Jan, S Faridullah, S Sherani, N Jahan. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. Sci World J 2017. [Google Scholar] [Crossref]
- MS Auwal, S Saka, IA Mairiga, KA Sanda, A Shuaibu, A Ibrahim. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Vet Res Forum 2014. [Google Scholar]
- B Singh, A Ahmad, V Pal. Evaluation of Antibacterial Activity and Phytochemical Screening of Azadirachtaindica Leaves Extracts Against Staphylococcus aureus. UK J Pharm Biosci 2015. [Google Scholar]
- VP Devmurari. Phytochemical screening study and antibacterial evaluation of SymplocosracemosaRoxb. Arch Appl Sci Res 2010. [Google Scholar]
- P Shrestha, S Adhikari, B Lamichhane, BG Shrestha. Phytochemical Screening of the Medicinal Plants of Nepal. IOSR J Environ Sci 2015. [Google Scholar]
- U Masih, R Shrimali, SMA Naqvi. Antibacterial Activity of Acetone and Ethanol Extracts of Cinnamon (Cinnamomumzeylanicum) and Ajowan (Trachyspermum ammi) on four Food Spoilage Bacteria. Int Res J Biol Sci 2012. [Google Scholar]
- B Shahidi. Evaluation of antibacterial properties of some medicinal plants used in Iran. J Ethno Pharmacol 2004. [Google Scholar]
- GJ Kaur, DS Arora. In vitro antibacterial activity of three plants belonging to the family Umbelliferae. Int J Antimicrob Agents 2008. [Google Scholar]
- Introduction
- Materials and Methods
- Result and Discussion
- Antibacterial activity of methanol extract of seeds of Trachyspermum ammi.
- Antibacterial activity of ethanol extract of Trachyspermum ammi
- Antibacterial activity of acetone extract of Trachyspermum ammi
- Phytochemical analysis of Trachyspermum ammi extract
- Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
- Acknowledgement
